Abstract
Objectives
To examine priorities for health status improvement in patients with active rheumatoid arthritis (RA) during anti‐tumour necrosis factor (TNF) treatment.
Methods
Data were used from 173 patients with RA starting treatment with TNF‐blocking agents. Outcome measures included assessment of health status with the Arthritis Impact Measurement Scales 2 (AIMS2) at baseline and after 3 and 12 months. The AIMS2 contains a priority list from which patients are asked to select from 12 areas of health the 3 in which they would most like to see improvement.
Results
After 1 year of treatment, 10 out of 12 areas of health on the AIMS2 were significantly improved. The most commonly selected priorities for improvement at baseline were pain (88%), hand and finger function (57%), walking and bending (42%), mobility (33%), and work (29%). At group level, this priority ranking remained largely unchanged during treatment. After adjustment for multiple comparisons, only pain was selected significantly less often at 3 and 12 months (71% at both assessments). Within individual patients, however, priorities often changed. Changes in the priority of pain were related to the achieved level of patient‐perceived pain and disease activity.
Conclusions
This study shows that, at the group level, patients' priorities for improvement are fairly stable during 12 months of anti‐TNF therapy, despite major improvements in health status. Although pain reduction becomes somewhat less important, it remains the most commonly selected priority. In contrast, individual patient priorities are not stable over the course of treatment and appear to be associated with differences in disease state.
Keywords: rheumatoid arthritis, health status, pain, patient priorities
Rheumatoid arthritis (RA) is a common chronic inflammatory disease that greatly affects patients' physical, psychological and social wellbeing.1,2,3,4 Over the years, various questionnaires have become available for measuring health status in patients with RA and multidimensional assessment of health status has now become common in clinical trials of RA. Clinicians or investigators usually determine the relative importance of these different dimensions of health. However, not all aspects of health are equally important to different patients5,6 and patients' and doctors' perceptions of important health status outcomes may differ considerably.7,8,9,10
To accurately measure health status from the patient's perspective, it is essential to identify the aspects of health that patients would most like to see improved. Previous studies that have explored patient perceptions of the relative importance of improving different health aspects generally indicate that patients with RA consider pain and physical disabilities to be the most important targets for treatment.7,11,12,13,14,15,16,17,18 However, it has been suggested that the relative importance of specific outcomes is not stable over the course of time or treatment.6,19 During a flare, for example, pain reduction may be the most important priority, whereas other areas of health are more important during stable disease.20,21
To date, no studies have examined longitudinal changes in patients' priorities for improvement. One recent study examined 7‐year changes in priorities for improvement in two cross‐sectional RA cohorts.22 Although all aspects of health had improved, patients' priorities for improvement remained mostly unchanged. This suggests that priorities for improvement are quite stable over time and not clearly associated with achieved improvements in health status. However, the authors performed a cross‐sectional comparison on two partially overlapping populations, thus complicating the interpretation of the results.23 Moreover, the observed improvements in health status were only minimal.23
The goals of the present study were to investigate the priorities for health status improvement in a cohort of patients with RA with high disease activity beginning tumour necrosis factor (TNF)‐blocking treatment, and to examine changes in these priorities after 3 and 12 months.
Methods
Patients and study design
The data for this study were collected as part of the ongoing Dutch Rheumatoid Arthritis Anti‐TNF Monitoring (DREAM) study, a register that started in April 2003 to prospectively monitor and evaluate the use of anti‐TNF in patients with RA in 12 hospitals in the Netherlands. In this study, all patients with RA starting on anti‐TNF are evaluated every 3 months. Inclusion criteria for the DREAM study are a diagnosis of RA,24 active disease (Disease Activity Score 28 (DAS28) >3.2),25 previous treatment with at least two anti‐rheumatic drugs including methotrexate at an optimum dose, or intolerance to methotrexate and no previous treatment with anti‐TNF agents.
For this study, we used data from a subset of centres that included the following measures at baseline and at the 3‐month and 12‐month follow‐up assessments: Health Assessment Questionnaire Disability Index (HAQ‐DI),26,27 Rheumatoid Arthritis Disease Activity Index (RADAI),28,29 the 100 mm Visual Analogue Scale for General Health (VAS‐GH) and the Arthritis Impact Measurement Scales 2 (AIMS2).15,17
Measures
The HAQ‐DI contains 20 items measuring physical disabilities over the past week in eight categories of daily living: dressing, arising, eating, walking, hygiene, reach, grip, and common daily activities.26,27 The HAQ was scored using the standard Disability Index, which takes into account the use of aids and devices. The HAQ‐DI yields a score from 0 to 3, with higher scores indicating more disability.
The RADAI is a 5‐item questionnaire for disease activity that asks patients to rate their global disease activity in the past 6 months, current disease activity in terms of swollen and tender joints, current arthritis pain, current duration of morning stiffness and number of tender joints in a joint list.28,29 The first three items were rated on 11‐point numerical rating scales. The combined RADAI score ranges from 0 to 10, with higher scores indicating more disease activity.
The VAS‐GH is a 100 mm horizontal line ranging from 0 (best) to 100 (worst). Patients were asked to rate their current general health.
The AIMS2 is a disease‐specific questionnaire designed to measure various components of health status in patients with arthritis.15,17 The core part of the questionnaire contains 57 items that are categorised in 12 scales representing different areas of health. The scales can be combined into five summary component scores: physical (mobility level, walking and bending, hand and finger function, arm function, self‐care, household tasks), affect (level of tension, mood), symptom (arthritis pain), social interaction (social activity, support from family and friends) and role (work). The scores on each scale or component range from 0 to 10, with higher scores representing poorer health status. Additionally, the AIMS2 contains sections on patient satisfaction with the 12 areas of health, effect of arthritis on each area of health, priorities for improvement, general perceptions of current and future health, and medical and demographic characteristics. The priority list (item 60) asks patients to select from 12 areas of health the 3 in which they would most like to see improvement. General satisfaction with current health (item 62) is assessed with a single‐item Likert scale ranging from very satisfied (1) to very dissatisfied (5). For the analyses, responses to this item were dichotomised into “satisfied” (very satisfied, somewhat satisfied) versus “not satisfied” (neither satisfied nor dissatisfied, somewhat dissatisfied, very dissatisfied).
Statistical analysis
Descriptive statistics were used to describe demographic and clinical characteristics and scores on outcome measures. Continuous data are presented as means with 95% confidence intervals (CI). Categorical data are presented as proportions with exact 95% CI for binomial distributions when appropriate.30
Paired two‐tailed t tests with Bonferroni correction for multiple comparisons were used to compare differences in means of patient‐reported outcomes between baseline and the 3‐month and 12‐month follow‐ups. For each area of health listed in question 60 from the AIMS2, changes in the proportions of patients who listed this area as a priority for improvement at baseline and at the 3‐month and 12‐month assessments were analysed using McNemar tests with Yates continuity correction and Bonferroni adjustment for multiple comparisons.
Results
Between April 2003 and November 2004, 226 patients were enrolled in this part of the study. Of these patients, 173 (77%) completed the AIMS2 at baseline and at the 3‐month and 12‐month follow‐ups. There were no significant differences in baseline age, gender, disease duration, DAS28 scores or Steinbrocker functional class31 distribution between patients who did and those who did not complete all three AIMS2 questionnaires (data not shown). Data from patients who did not complete all three AIMS2 questionnaires were excluded from further analyses.
Of the included 173 patients, 70% were women. Mean (95% CI) age and disease duration at study entry were 53.2 (51.3 to 55.2) years and 9.9 (8.5 to 11.3) years, respectively. Assessment of disease severity at baseline generally indicated severe RA, with a DAS28 score of 5.5 (5.3 to 5.7). According to the Steinbrocker functional classification, 7% of the patients were classified as class I, 81% as class II and 12% as class III.
Three months after the start of treatment, patient‐reported physical disabilities, disease activity and general health were significantly improved (table 1), as were most aspects of health as measured with the AIMS2. Improvements were most pronounced for physical aspects of health. All improvements remained relatively stable at the 12‐month follow‐up, with the gradual improvements in self‐care, work and level of tension on the AIMS2 becoming significantly different from baseline after 12 months. The proportion of patients who were satisfied with their current general health also significantly increased from 20.8% (15.0 to 27.6) at baseline to a relatively stable 48.6% (40.9 to 56.3, McNemar test, p<0.001) at the 3‐month follow‐up and 51.4% (43.7 to 59.1) at the 12‐month follow‐up.
Table 1 Patient‐reported outcomes at baseline and the 3‐month and 12‐month follow‐ups.
| Outcome, mean (95% CI) | |||
|---|---|---|---|
| Baseline | 3 months | 12 months | |
| HAQ‐DI (range 0 to 3) | 1.4 (1.3 to 1.5) | 1.1 (1.0 to 1.2)† | 1.1 (1.0 to 1.2)† |
| RADAI (range 0 to 10) | 5.5 (5.2 to 5.8) | 3.7 (3.4 to 4.0)† | 3.2 (2.9 to 3.5)† |
| VAS‐GH (range 0 to 100) | 58.0 (54.4 to 61.6) | 42.5 (38.8 to 46.1)† | 38.9 (35.1 to 42.6)† |
| AIMS2 (range 0 to 10) | |||
| Mobility level | 2.5 (2.2 to 2.8) | 2.0 (1.7 to 2.3)† | 2.0 (1.7 to 2.3)† |
| Walking and bending | 5.8 (5.5 to 6.1) | 4.8 (4.4 to 5.1)† | 4.6 (4.3 to 5.0)† |
| Hand and finger | 4.3 (3.9 to 4.6) | 3.2 (2.9 to 3.5)† | 3.1 (2.8 to 3.4)† |
| Arm function | 2.8 (2.5 to 3.1) | 1.8 (1.6 to 2.1)† | 1.7 (1.4 to 2.0)† |
| Self‐care | 1.3 (1.1 to 1.6) | 1.0 (0.7 to 1.2) | 0.8 (0.6 to 1.1)† |
| Household tasks | 2.6 (2.3 to 3.0) | 2.0 (1.7 to 2.3)† | 2.1 (1.7 to 2.4)† |
| Social activities | 5.1 (4.9 to 5.3) | 4.9 (4.8 to 5.1) | 4.8 (4.7 to 5.0) |
| Support from family | 2.7 (2.4 to 3.1) | 2.6 (2.3 to 3.0) | 2.5 (2.1 to 2.9) |
| Arthritis pain | 6.7 (6.4 to 7.1) | 4.6 (4.2 to 4.9)† | 4.5 (4.1 to 4.9)† |
| Work* | 4.4 (3.6 to 5.1) | 3.6 (2.9 to 4.3) | 3.1 (2.4 to 3.7)† |
| Level of tension | 3.8 (3.5 to 4.1) | 3.5 (3.2 to 3.7) | 3.2 (3.0 to 3.5)† |
| Mood | 3.2 (3.0 to 3.5) | 2.6 (2.3 to 2.8)† | 2.5 (2.3 to 2.7)† |
AIMS2, Arthritis Impact Measurement Scales; HAQ‐DI, Health Assessment Questionnaire Disability Index; RADAI, Rheumatoid Arthritis Disease Activity Index; VAS‐GH, visual analogue scale for general health.
*n = 59.
†Significantly different from baseline after Bonferroni correction for multiple comparisons (p<0.05/45). No significant differences between 3‐month and 12‐month follow‐ups.
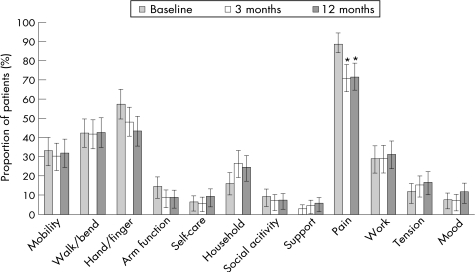
The proportions of patients who selected the different areas of health as a priority for improvement during the study period are shown in table 2. At baseline, arthritis pain was the major priority for improvement, selected by about 90% of the patients. Other priorities were various aspects of physical function, including hand and finger function, walking and bending, and mobility. Almost one‐third of the patients chose health status related to work as an important priority. Other aspects of health, including all psychosocial aspects, were selected by <20% of the patients.
Table 2 Patients who listed various areas of health from the AIMS2 as a priority for improvement at baseline and the 3‐month and 12‐month follow‐ups.
| Outcome, n (%) (exact 95% binomial CI) | |||
|---|---|---|---|
| Baseline | 3 months | 12 months | |
| Mobility level | 57 (32.9) ( 26.0 to 40.5) | 52 (30.1) ( 23.3 to 37.5) | 55 (31.8) ( 24.9 to 39.3) |
| Walking and bending | 73 (42.2) ( 34.7 to 49.9) | 72 (41.6) ( 34.2 to 49.3) | 74 (42.8) ( 35.3 to 50.5) |
| Hand and finger function | 99 (57.2) ( 49.5 to 64.7) | 83 (48.0) ( 40.3 to 55.7) | 75 (43.4) ( 35.9 to 51.1) |
| Arm function | 25 (14.5) ( 9.6 to 20.6) | 15 (8.7) ( 4.9 to 13.9) | 15 (8.7) ( 4.9 to 13.9) |
| Self‐care | 11 (6.4) ( 3.2 to 11.1) | 10 (5.8) ( 2.8 to 10.4) | 16 (9.2) ( 5.4 to 14.6) |
| Household tasks | 28 (16.2) ( 11.0 to 22.5) | 46 (26.6) ( 20.2 to 33.8) | 42 (24.3) ( 18.1 to 31.4) |
| Social activities | 16 (9.2) ( 5.4 to 14.6) | 12 (6.9) ( 3.6 to 11.8) | 13 (7.5) ( 4.1 to 12.5) |
| Support from family | 5 (2.9) ( 0.9 to 6.7) | 8 (4.6) ( 2.0 to 8.9) | 10 (5.8) ( 2.8 to 10.4) |
| Arthritis pain | 153 (88.4) ( 82.7 to 92.8) | 122 (70.5) ( 63.1 to 77.2) | 123 (71.1) ( 63.7 to 77.7) |
| Work | 50 (28.9) ( 22.3 to 36.3) | 50 (28.9) ( 22.3 to 36.3) | 54 (31.2) ( 24.4 to 38.7) |
| Level of tension | 20 (11.6) ( 7.2 to 17.3) | 26 (15.0) ( 10.1 to 21.2) | 29 (16.8) ( 11.5 to 23.2) |
| Mood | 13 (7.5) ( 4.1 to 12.2) | 12 (6.9) ( 3.6 to 11.8) | 20 (11.6) ( 7.2 to 17.3) |
AIMS2, Arthritis Impact Measurement Scales.
At the group level, this priority ranking remained mostly unchanged during treatment (fig 1). At both the 3‐month and 12‐month follow‐ups, the top six priorities of improvement remained the same, with only minor shifts occurring within the less commonly selected areas of health, such as level of tension and arm function. Some changes were seen in the frequency in which individual areas of health were selected. Most notable were the decreased priority of improvement in hand and finger function and pain and the increased priority of household tasks. However, after Bonferroni correction for multiple comparisons, only the decreased priority of pain reduction retained significance. Although arthritis pain remained the major priority for improvement during the study period, the proportion of patients who selected this health area significantly decreased at the 3‐month follow‐up and remained stable thereafter.
Figure 1 Proportion (exact 95% binomial CI) of patients who listed the different areas of health as a priority for improvement at baseline and the 3‐month and 12‐month follow‐ups. *Significantly different from baseline after Bonferroni correction for multiple comparisons (p<0.05/36); no significant differences between 3‐month and 12‐month follow‐ups.
Although priorities for improvement during treatment were fairly stable at the group level, there was considerable intraindividual variation in priorities over time. The proportion of patients who changed the priority classification of an aspect of health (from either no priority to priority or from priority to no priority) at the two follow‐ups ranged between 6.4% and 34.7% for the different aspects of health (see supplementary table W1, available at http://ard.bmjjournals.com/supplemental). From the patients who selected exactly three priorities both at baseline and after 3 months, only 19% selected the same list of priorities on both occasions (12% between 3 and 12 months), 56% made one change in their priority list (56% between 3 and 12 months), 23% selected two new priorities (29% between 3 and 12 months) and 2% selected none of the priorities from their previous list (3% between 3 and 12 months).
Individual changes in the priority status of pain at 3 and 12 months were related to concurrent levels of pain and disease activity (table 3). Patients who dropped pain from their priority list reported a significantly lower level of pain and disease activity than patients for whom pain remained a priority for improvement. Conversely, patients who changed pain from no priority to priority reported significantly more pain and disease activity than patients who continued to exclude it as a priority. Changes in the priority of pain improvement were not associated with different scores on the VAS‐GH, HAQ‐DI and the physical, affect, social and role components of the AIMS2.
Table 3 Association between changes in the priority of pain and mean levels (95% CI) of patient‐perceived pain and disease activity.
| 3 months | 12 months | |||||||
|---|---|---|---|---|---|---|---|---|
| P/P (n = 112) | NP/P (n = 10) | P/NP (n = 41) | NP/NP (n = 10) | P/P (n = 96) | NP/P (n = 27) | P/NP (n = 26) | NP/NP (n = 24) | |
| AIMS2 Pain | 5.0 (4.6 to 5.4) | 6.2 (4.3 to 8.0)* | 3.4 (2.7 to 4.1)† | 2.9 (1.5 to 4.3) | 5.2 (4.7 to 5.6) | 5.0 (4.2 to 5.7)* | 3.7 (2.6 to 4.7)† | 2.3 (1.7 to 3.0) |
| RADAI | 3.9 (3.5 to 4.2) | 5.3 (3.7 to 6.8)* | 2.9 (2.3 to 3.5)† | 3.3 (2.3 to 4.3) | 3.6 (3.2 to 4.0) | 3.5 (2.8 to 4.3)* | 2.6 (1.8 to 3.3)† | 2.0 (1.2 to 2.7) |
AIMS2, Arthritis Impact Measurement Scales;NP/NP, no priority to no priority; NP/P, no priority to priority; P/NP, priority to no priority; P/P, priority to priority; RADAI, Rheumatoid Arthritis Disease Activity Index.
*Significantly different from NP/NP (p<0.05).
†Significantly different from P/P (p<0.05).
Discussion
This is the first study to examine the longitudinal course of patients' priorities for improvement in a cohort of patients with RA during anti‐TNF treatment. The results suggest that, at a group level, patients' priority rankings are fairly stable during 1 year of treatment, despite major improvements in health status. Although pain reduction becomes somewhat less important after 3 months of treatment, it remains the highest priority of improvement for patients with RA. At the individual patient level, however, priorities are not stable and appear to be associated with changes in disease state.
Our finding that improvements in pain and aspects of physical function are of primary importance to patients with RA is consistent with previous studies,7,11,12,13,14,15,16,17,18 although some studies have suggested that physical disability or loss of mobility and dependency on others may be more important problems than pain itself.32,33,34 In 1985, Gibson and Clark12 found that 47% of 120 randomly selected patients with RA rated pain relief and 21% rated increased physical activity as the most desirable objectives of their treatment. A similar study of 250 patients with rheumatic disease showed that 66% of the included 120 patients with RA ranked pain and 22% ranked disability as the most important symptoms to be treated.14 Both studies, however, focused only on physical aspects of RA and did not include any psychological or social dimensions of health.
Another study of 79 patients with various rheumatic diseases that did include psychosocial aspects of health reported that being free of pain was the symptom status outcome that the majority of patients (63%) identified as the most important outcome of treatment.7 The feeling of being in control was the mental health outcome rated most important by the largest number of the patients (42%), activities involving the legs the most important physical health outcome (38%) and working at a job or around the house the most important social health outcome (62%). However, as the various dimensions of health were examined separately, this study did not assess the relative weight or importance attached to the physical, psychological and social aspects of health.
Several studies have used the more comprehensive priority list from the AIMS2 to describe patients' priorities for improvement in mostly cross‐sectional RA populations. In the validation study of the original AIMS2, 62% of 299 patients with RA designated pain as a priority area.15 Next were walking and bending (49%), hand and finger function (47%), and household tasks (30%). Another study reported comparable priorities in 92 patients with RA for pain (67%), walking and bending (41%), and hand and finger function (42%), but also a high priority for mobility (53%).11 Two previous studies in Dutch patients with RA found similar high priorities for pain (74% and 75%, respectively), walking and bending (52% and 46%), and hand and finger function (41% and 38%), although in both studies household tasks was selected by <20% of the patients.17,18 Minnock et al16 found that 68% of 58 women with RA prioritised pain as an area of health needing improvement. Surprisingly, walking and bending (25%) and hand and finger function (25%) were less often selected as a priority in this study, whereas household tasks and mood were selected by 44% and 26%, respectively. Finally, in a recent study of 1024 patients with RA, pain was selected by 69%, walking and bending by 33%, and hand and finger function by 24% of the patients.
The distributions of priorities in our study are reasonably consistent with these studies, with the exception of the relatively high priorities for hand and finger function and pain at baseline. During treatment, however, the proportion of patients that selected hand and finger function and pain as a priority decreased to comparable levels, as observed in the previous observations. Nonetheless, one other notable difference between priorities observed in this study and most other studies remained visible at all three assessments. The patients in this study more commonly selected aspects related to work as a priority for improvement. This may be related to cultural differences in the importance of being able to work, since a previous study in Dutch patients with RA also found a high priority for work‐related aspects of health.18
This study also confirms the finding of Heiberg et al22 that pain improvement remains the top priority for patients with RA over time, despite marked improvements in its intensity. Contrary to their findings, however, this study did show a significant decrease in the number of patients that selected pain as an area for improvement after 3 months of treatment. Moreover, within individual patients, priorities often changed and longitudinal changes in the priority for pain improvement were associated with the achieved level of pain and disease activity at the respective follow‐up assessments. This gives some support to the idea that the importance of particular outcomes to patients may vary during different disease states and that existing measures may be enhanced by taking account of these variations in priorities.20,21 However, as the current sample size was too small to permit extensive subgroup analyses, this association has yet to be confirmed for other areas of health.
The finding that pain remained the most selected priority for improvement during treatment may indicate that the improvements in pain (although significant at a group level) were still not large enough to lead to an “acceptable” level of pain for most individual patients. Although no established standards exist for a patient‐acceptable symptom state on the AIMS2, the results showed that patients who dropped pain as a priority for improvement had a mean pain score of about 3.5 on the AIMS2 pain scale. However, at both the 3‐month and 12‐month follow‐ups, <40% of the patients actually achieved a pain score below 3.5.
Limitations
The study has some limitations. The first concerns the use of the priority list of the AIMS2 questionnaire to measure priorities for improvement. This priority list may not include all aspects of health that are important to patients with RA. For instance, the list does not include fatigue and general wellbeing, which have been identified by patients as important outcomes of treatment.20,35,36,37 In addition, different dimensions of health are represented by different numbers of items on the priority list, which may have influenced the results. Finally, despite clear instructions to the contrary accompanying the priority list, it was possible for patients to select >3 priorities for improvement. However, as <5% of the patients selected >3 items at the different assessments, this is not likely to have significantly affected the results.
Another limitation concerns the generalisability of the current findings. Medical interventions such as anti‐TNF treatment are primarily aimed at improving the pathophysiological processes of inflammation. Consequently, primary signs and symptoms such as pain, swollen and tender joints and impaired function are most likely to improve. Although theoretically, psychosocial aspects are induced by the disease process and should improve also in case of effective therapy, specific psychosocial interventions may very well result in different priority distributions.
Finally, as the duration of this study was limited to 1 year, no causal conclusions can be drawn about long‐term changes in patients' priorities. A recent qualitative study in patients with RA suggested that the relative importance of different aspects of health changes as the disease progresses.20 Patients reported that pain was most important in their early disease, and that mobility and independence were more important in later disease. However, to date there is no quantitative evidence that disease duration has long‐term effects on patients' priorities for outcome improvement.21
Conclusion
This study suggests that patients' priorities for improvement are fairly stable over time, although individual priorities can change as a result of effective treatment. Pain reduction remains the most important priority for patients with RA, even after 1 year of anti‐TNF treatment.
Supplementary material is available on the ARD website at http://ard.bmj.com/supplemental
Copyright © 2007 BMJ Publishing Group and European League Against Rheumatism
Supplementary Material
Acknowledgements
We thank T van Gaalen, W Kievit and P Welsing for their contribution to the organisation of the study and data management. We thank the following rheumatologists and research nurses for their assistance in patient recruitment and data collection: J Alberts, C Allaart, A ter Avest, P Barrera Rico, T Berends, H Bernelot Moens, K Bevers, C Bijkerk, A van der Bijl, J de Boer, A Boonen, E ter Borg, E Bos, Botha, A Branten, F Breedveld, H van den Brink, J Bürer, G Bruyn, H Cats, M Creemers, J Deenen, C De Gendt, K Drossaers‐Bakker, A van Ede, A Eijsbouts, S Erasmus, M Franssen, I Geerdink, M Geurts, E Griep, E de Groot, C Haagsma, H Haanen, JHarbers, A Hartkamp, J Haverman, H van Heereveld, van de Helm‐van Mil, I Henkes, S Herfkens, M Hoekstra, K van de Hoeven, DM Hofman, M Horbeek, F van den Hoogen, P M Houtman, T Huizinga, H Hulsmans, P Jacobs, T Jansen, M Janssen, M Jeurissen, A de Jong, de Jong, M Kleine Schaar, G Kloppenburg, H Knaapen, P Koelmans, Kortekaas, B Kraft, A Krol, M Kruijssen, D Kuiper‐Geertsma, I Kuper, R Laan, J van de Laan, J van Laar, P Lanting, H Lim, S van der Linden, A Mooij, J Moolenburgh, N Olsthoorn, P van Oijen, van Oosterhout, J Oostveen, P van 't Pad Bosch, K Rasing, K Ronday, D de Rooij, L Schalkwijk, P Seys, P de Sonnaville, A Spoorenberg, A Stenger, G Steup, W Swen, J Terwiel, M van der Veen, M Veerkamp, C Versteegden, H Visser, C Vogel, M Vonk, H Vonkeman, AWestgeest, H van Wijk, N Wouters.
Abbreviations
AIMS2 - Arthritis Impact Measurement Scales
DAS - Disease Activity Score
DREAM - Dutch Rheumatoid Arthritis Anti‐TNF Monitoring
HAQ‐DI - Health Assessment Questionnaire Disability Index
RA - rheumatoid arthritis
RADAI - Rheumatoid Arthritis Disease Activity Index
TNF - tumour necrosis factor
VAS‐GH - Visual Analogue Scale for General Health
Footnotes
Funding: This study was funded by an unrestricted educational grant by Schering‐Plough and CVZ (the Dutch Health Care Insurance Board).
Supplementary material is available on the ARD website at http://ard.bmj.com/supplemental
References
- 1.Anderson K O, Bradley L A, Young L D, McDaniel L K, Wise C M. Rheumatoid arthritis: review of psychological factors related to etiology, effects and treatment. Psychol Bull 198598358–387. [PubMed] [Google Scholar]
- 2.Lapsley H M, March L M, Tribe K L, Cross M J, Courtenay B G, Brooks P M. Living with rheumatoid arthritis: expenditures, health status and social impact on patients. Ann Rheum Dis 200261818–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Meenan R F, Yelin E H, Nevitt M, Epstein W V. The impact of chronic disease: a sociomedical profile of rheumatoid arthritis. Arthritis Rheum 198124544–549. [DOI] [PubMed] [Google Scholar]
- 4.Yelin E, Lubeck D, Holman H, Epstein W. The impact of rheumatoid arthritis and osteoarthritis: the activities of patients with rheumatoid arthritis and osteoarthritis compared to controls. J Rheumatol 198714710–717. [PubMed] [Google Scholar]
- 5.Gill T M, Feinstein A R. A critical appraisal of the quality of quality‐of‐life measurements. JAMA 1994272619–626. [PubMed] [Google Scholar]
- 6.O'Boyle C A, McGee H, Hickey A, O'Malley K, Joyce C R. Individual quality of life in patients undergoing hip replacement. Lancet 19923391088–1091. [DOI] [PubMed] [Google Scholar]
- 7.Kwoh C K, Ibrahim S A. Rheumatology patient and physician concordance with respect to important health and symptom status outcomes. Arthritis Rheum 200145372–377. [DOI] [PubMed] [Google Scholar]
- 8.Rothwell P M, McDowell Z, Wong C K, Dorman P J. Doctors and patients don't agree: cross sectional study of patients' and doctors' perceptions and assessments of disability in multiple sclerosis. BMJ 19973141580–1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hewlett S A. Patients and clinicians have different perspectives on outcomes in arthritis. J Rheumatol 200330877–879. [PubMed] [Google Scholar]
- 10.Hewlett S, Smith A P, Kirwan J R. Values for function in rheumatoid arthritis: patients, professionals and public. Ann Rheum Dis 200160928–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Archenholtz B, Bjelle A. Reliability, validity and sensitivity of a Swedish version of the revised and expanded Arthritis Impact Measurement Scales (AIMS2). J Rheumatol 1997241370–1377. [PubMed] [Google Scholar]
- 12.Gibson T, Clark B. Use of simple analgesics in rheumatoid arthritis. Ann Rheum Dis 19854427–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heiberg T, Kvien T K. Preferences for improved health examined in 1,024 patients with rheumatoid arthritis: pain has highest priority. Arthritis Rheum 200247391–397. [DOI] [PubMed] [Google Scholar]
- 14.McKenna F, Wright V. Pain and rheumatoid arthritis. Ann Rheum Dis 198544805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meenan R F, Mason J H, Anderson J J, Guccione A A, Kazis L E. AIMS2. The content and properties of a revised and expanded Arthritis Impact Measurement Scales health status questionnaire. Arthritis Rheum 1992351–10. [DOI] [PubMed] [Google Scholar]
- 16.Minnock P, Fitzgerald O, Bresnihan B. Quality of life, social support and knowledge of disease in women with rheumatoid arthritis. Arthritis Rheum 200349221–227. [DOI] [PubMed] [Google Scholar]
- 17.Riemsma R P, Taal E, Rasker J J, Houtman P M, van Paassen H C, Wiegman O. Evaluation of a Dutch version of the AIMS2 for patients with rheumatoid arthritis. Br J Rheumatol 199635755–760. [DOI] [PubMed] [Google Scholar]
- 18.Taal E, Rasker J J, Evers A W, Kraaimaat F W, Lanting P J H, Jacobs J W. Which priorities have rheumatoid arthritis (RA) patients for their health status improvement? (Abstract). Arthritis Rheum 199740(Suppl 1)S231–S240. [Google Scholar]
- 19.Carr A J, Higginson I J. Are quality of life measures patient centred? BMJ 20013221357–1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carr A, Hewlett S, Hughes R, Mitchell H, Ryan S, Carr M.et al Rheumatology outcomes: the patient's perspective. J Rheumatol 200330880–883. [PubMed] [Google Scholar]
- 21.Kirwan J R, Hewlett S E, Heiberg T, Hughes R A, Carr M, Hehir M.et al Incorporating the patient perspective into outcome assessment in rheumatoid arthritis‐progress at OMERACT 7. J Rheumatol 2005322250–2256. [PubMed] [Google Scholar]
- 22.Heiberg T, Finset A, Uhlig T, Kvien T K. Seven year changes in health status and priorities for improvement of health in patients with rheumatoid arthritis. Ann Rheum Dis 200564191–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boonen A, Landewe R. Health status in rheumatoid arthritis over 7 years. Ann Rheum Dis 200564173–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arnett F C, Edworthy S M, Bloch D A, McShane D J, Fries J F, Cooper N S.et al The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum 198831315–324. [DOI] [PubMed] [Google Scholar]
- 25.Prevoo M L, van 't Hof M A, Kuper H H, van Leeuwen M A, van de Putte L B, van Riel P L. Modified Disease Activity Scores that include twenty‐eight‐joint counts: development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis Rheum 19953844–48. [DOI] [PubMed] [Google Scholar]
- 26.Fries J F, Spitz P, Kraines R G, Holman H R. Measurement of patient outcome in arthritis. Arthritis Rheum 198023137–145. [DOI] [PubMed] [Google Scholar]
- 27.Siegert C E, Vleming L J, Vandenbroucke J P, Cats A. Measurement of disability in Dutch rheumatoid arthritis patients. Clin Rheumatol 19843305–309. [DOI] [PubMed] [Google Scholar]
- 28.Stucki G, Liang M H, Stucki S, Bruhlmann P, Michel B A. A self‐administered rheumatoid arthritis disease activity index (RADAI) for epidemiologic research. Psychometric properties and correlation with parameters of disease activity. Arthritis Rheum 199538795–798. [DOI] [PubMed] [Google Scholar]
- 29.Fransen J, van Halm V P, Nurmohamed M T, van Riel P L, de Ryck Y, Dijkmans B A. Validity of the rheumatoid arthritis disease activity index (RADAI) in a two year open label study with leflunomide. Ann Rheum Dis 200564(suppl 3)194 [Google Scholar]
- 30.Bland M.An introduction to medical statistics, 3rd ed. Oxford: Oxford University Press 2000
- 31.Steinbrocker O, Traeger C H, Battman R G. Therapeutic criteria in rheumatoid arthritis. JAMA 1949140659–662. [DOI] [PubMed] [Google Scholar]
- 32.Chamberlain M A, Buchanan J M, Hanks H. The arthritic in an urban environment. Ann Rheum Dis 19793851–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cornelissen P G, Rasker J J, Valkenburg H A. The arthritis sufferer and the community: a comparison of arthritis sufferers in rural and urban areas. Ann Rheum Dis 198847150–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Taal E, Rasker J J, Seydel E R, Wiegman O. Health status, adherence with health recommendations, self‐efficacy and social support in patients with rheumatoid arthritis. Patient Educ Couns 19932063–76. [DOI] [PubMed] [Google Scholar]
- 35.Kirwan J, Heiberg T, Hewlett S, Hughes R, Kvien T, Ahlmen M.et al Outcomes from the Patient Perspective Workshop at OMERACT 6. J Rheumatol 200330868–872. [PubMed] [Google Scholar]
- 36.Ahlmen M, Nordenskiold U, Archenholtz B, Thyberg I, Ronnqvist R, Linden L.et al Rheumatology outcomes: the patient's perspective. A multicentre focus group interview study of Swedish rheumatoid arthritis patients. Rheumatology (Oxford) 200544105–110. [DOI] [PubMed] [Google Scholar]
- 37.Hewlett S, Carr M, Ryan S, Kirwan J, Richards P, Carr A.et al Outcomes generated by patients with rheumatoid arthritis: how important are they? Musculoskeletal Care 20053131–142. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.