Abstract
Background and objective
Treatment‐limiting infusion reactions to infliximab have not been fully explained in rheumatoid arthritis patients. Our main objective is to investigate the role of daily oral glucocorticoids use on such reactions.
Method
Forty‐three patients with immediate‐type infusion reactions were identified in a large registry‐based cohort. These patients were then compared with the entire cohort (n = 639) and, in a separate analysis, to a nested matched control group (n = 43). The following base‐line variables were compared: use of oral glucocorticoids, health‐assessment questionnaire, 28‐joint count‐based disease activity score, duration of disease and number of failed disease‐modifying antirheumatic drugs.
Results
The proportion of infusions associated with infusion reactions decreased significantly during the study period (p = 0.0024). Fifty per cent of the patients in the cohort were treated with daily low‐dose glucocorticoids at baseline. 15/326 (4.6%) patients had an infusion reaction as compared with 28/324 (8.6%) of patients without glucocorticoid treatment (p = 0.057). In the matched comparison, 15/43 (35%) of the cases were on low‐dose glucocorticoids as compared with 27/43 (64%) of the controls (p = 0.017). The use of low‐dose glucocorticoids was associated with a significantly lower risk for a treatment‐limiting infusion reactions in a Kaplan–Meier analysis (p = 0.04). The number needed to treat to prevent a treatment‐limiting infusion reaction was 25 (95% CI: 13 to 527) in the cohort.
Conclusion
The use of daily low‐dose glucocorticoids is associated with a lower risk for treatment‐limiting infusion reactions to infliximab. Overall, treatment‐limiting infusion reactions have become significantly less common during the past 5 years.
Infliximab, together with the other tumour necrosis factor antagonists, etanercept and adalimumab, has become one of the most important drugs in the treatment of rheumatoid arthritis (RA). Clinical efficacy for infliximab has been shown in several clinical trials1,2,3 as well as in registry studies.4,5 Registry studies have also been useful in detecting adverse events for all three of the tumour necrosis factor (TNF) antagonists. Cohort studies provide useful information about the true incidence of adverse events in clinical practice. The fact that patients in clinical practice may differ considerably from those in clinical trials in comorbidity and concurrent drug treatment, for example, further emphasises the importance of these registries.6,7,8,9,10,11
The occurrence of infusion reactions is an area of special consideration for infliximab.
In RA, approximately 20% of infliximab‐treated patients in all clinical trials experienced an infusion reaction, with 3% annually discontinuing the treatment with infliximab caused by such a reaction.1 Two objectives led us to investigate this issue further: (1) that the real‐life frequency of treatment‐limiting infusion reactions might well be higher than that reported from clinical trials; and (2) that even a low annual frequency of such reactions might, over several years, come to represent a significant limitation of treatment with infliximab.
Infusion reactions and risk factors predicting such reactions have been studied more thoroughly in Crohn's disease than in RA. Crandall and Mackner found female gender and the use of immunosuppressive medications for less than 4 months to be risk factors for a reaction to a second infliximab infusion.12 A distant second infusion (⩾20 weeks from first infusion) was observed as a risk factor by Kugathasan et al.13 In contrast to the situation in CD, neither distance between infusions nor short use of immunosuppressive treatment is applicable in RA, since the vast majority of patients are treated with methotrexate (MTX) concurrently during treatment with infliximab. Thus, risk factors predicting treatment‐limiting infusion reactions in RA still remain unclassified. We have previously identified anticardiolipin antibodies as a risk factor for such infusion reactions.6 The aim of this study was to investigate the role of glucocorticoid use and other predictors of infusion reactions.
Methods
A total of 672 patients were treated with infliximab at Karolinska University Hospital at some occasion between 1999 and 2004. Data were obtained from the STURE (Stockholm TNF‐α follow‐up) registry and by searching patient records when data were missing. The STURE database collects efficacy and safety data for all patients starting biological treatments at all major hospitals in Stockholm, as part of the nationwide registry of AntiRheumatic Therapies In Sweden (ARTIS). The assessments are done at 0, 3, 6 and 12 months and annually thereafter, and include the ACR core outcomes, that is the 28 swollen and tender joint count, visual analogue scales for global health and for pain, the health assessment questionnaire (HAQ), erythrocyte sedimentation rate (ESR) and C‐reactive protein (CRP), and physician's global assessment of disease activity, and the 28‐joint count‐based disease activity score (DAS28).14,15
Forty‐three patients with immediate‐type infusion reactions, defined as an anaphylactic/anaphylactoid reaction and/or urticaria and itching that resulted in discontinuation of infliximab treatment, were compared with the entire cohort (n = 673) and, in a separate analysis, to a nested control group (n = 43) matched for gender, diagnosis and age.
Comparisons of the following baseline variables were made: HAQ, DAS28, visual analogue scale global and pain, number of swollen joints, number of tender joints, duration of disease at start of treatment, number of failed DMARDs before start of treatment and oral glucocorticoid dose.
The regional ethics committee approved the study (DNR: 03‐440).
Statistical analysis
Comparisons were by the Fisher's test for nominal variables, and by Mann–Whitney test and Wilcoxon test for all other variables. Survival on drug without infusion reaction was plotted according to Kaplan–Meier and compared by log rank test (Mantel–Cox). Logistic regression was used to determine odds ratios for continuous variables in the case‐control study. Statistical analysis was performed using Statview 5.0.1 software (SAS Corp, Cary, NC). The number needed to treat (NNT)16 was calculated over the study period.
Results
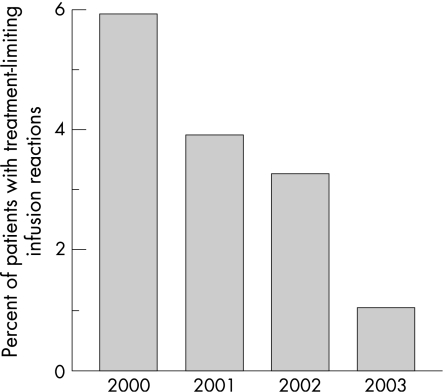
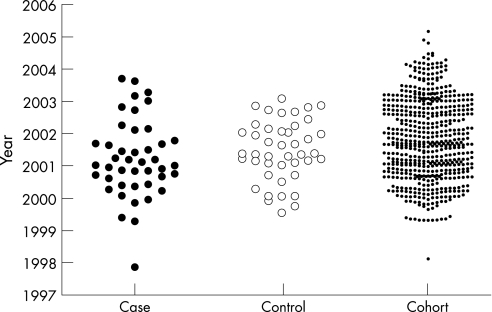
Baseline characteristics were collected for 673 patients treated with infliximab from 1999 to 2004. We identified 43 patients with treatment‐limiting infusion reactions to infliximab in this cohort. Demographic data for all patients are shown in table 1. These treatment‐limiting infusion reactions were recorded at various time points during therapy, slightly more frequent after five or more infusions. Infusion reactions represent the cause of approximately 15% of the discontinuations of this treatment, other causes being lack of efficacy, infections, etc. The proportion of infusions associated with infusion reactions decreased significantly from the year 2000 to the year 2003 (fig. 1). Nine out of 152 patients on infliximab (5.92%) had a treatment‐limiting infusion reaction in the year 2000 as compared with five out of 438 patients (1.14%) in 2003 (p = 0.0024). Figure 2 shows the yearly distribution of the start of treatment for cases, matched controls and cohort.
Table 1 Patient demographics at baseline.
| Cases n = 43 | Control n = 43 | Cohort* n = 630 | p value | ||
|---|---|---|---|---|---|
| Case vs control | Case vs cohort | ||||
| RA, n (%) | 32 (74) | 30 (70) | 437 (69) | ||
| Other rheumatic diagnoses, n (%) | 11 (26) | 13 (30)† | 129 (20)‡ | ||
| Female, n (%) | 33 (77) | 33 (77) | 437 (69) | ||
| Median age at first infusion, years (range) | 57 (21–84) | 53 (18–81) | 54 (17–87) | ||
| Disease duration, years | 13.5 (1.9) | 10.6 (1.3) | 11 (0.5) | 0.279 | 0.359 |
| ESR, mm | 42.2 (4.48) | 33.4 (3.7) | 34.5 (1.12) | 0.125 | 0.066 |
| CRP, mg/l | 39.0 (5.5) | 37.9 (4.9) | 35.2 (1.8) | 0.878 | 0.569 |
| DAS28 | 5.52 (0.24) | 5.75 (0.22) | 5.54 (0.06) | 0.793 | 0.550 |
| HAQ, baseline median (IQR§) | 1.75 (1.13–2.25) | 1.5 (0.63–2.25) | 1.38 (0.88–1.88) | 0.373 | 0.054 |
| No. of failed DMARDs | 3.7 (0.3) | 2.6 (0.2) | 3.8 (0.1) | 0.012 | 0.690 |
Data are given as mean (SEM) unless otherwise stated.
*Including controls; †of whom two controls with JCA were matched with cases with RA; ‡data missing for patients registered at private ward facilities; §IQR: interquartile range.
Figure 1 Percentage of patients experiencing a treatment‐limiting infusion by calendar year. The frequency declines sharply, and nine out of 152 patients on infliximab (5.92%, 95% CI: 2.7 to 10.7) had a treatment‐limiting infusion reaction in the year 2000 as compared with five out of 438 patients (1.14%, 95% CI: 0.4 to 2.6) in 2003 (p = 0.0024).
Figure 2 Patient demographics. Yearly distribution of start of treatment for cases, matched controls and cohort.
Most patients identified in our survey experienced several less severe infusion reactions before the discontinuation of treatment. The symptoms occurring during the last infusion are presented in table 2. The most common symptoms were shortness of breath followed by flushing and urticaria. Symptoms of immediate infusion reactions were most commonly treated by stopping the infusion and administering intravenous glucocorticoids and/or antihistamines (clemastine) (table 2). All patients who had experienced an infusion reaction were given prophylactic treatment before their next infusion. The most common pretreatment was intravenous steroids (hydrocortisone) and/or antihistamines (most commonly clemastine) 1 h before treatment (table 2). The decision to discontinue treatment with infliximab was taken by the responsible physician when the patient had a severe infusion reaction with or without pretreatment and/or had several infusion reactions in spite of pretreatment.
Table 2 Symptoms and treatment of treatment limiting infusion reactions.
| Symptom | No. of patients | Pharmacological treatment | No. of patients | Premedication and other prophylactic measures | No. of patients |
|---|---|---|---|---|---|
| Shortness of breath/chest pressure | 21 | Hydrocortisone intravenous | 12 | Clemastine (intravenous or per os) | 21 |
| Flush | 16 | Clemastine | 17 | Steroid (hydrocortisone intravenous) | 20 |
| Urticaria | 15 | Betamethasone | 11 | Initial slow infusion rate | 10 |
| Pruritus | 10 | Oxygen | 4 | Paracetamol/acetaminophen | 1 |
| Swelling of face | 6 | Hydration with saline | 6 | Loratadine | 1 |
| Shivers/cold sweat | 5 | Epinephrine | 4 | Diazepam | 1 |
| Nausea/vomiting | 3 | Diazepam | 3 | Prednisolone | 1 |
| Hypotension | 2 | Cetirizine | 1 | Hydration with saline | 1 |
| Tachycardia | 2 | Prednisolone | 1 | ||
| Increased pain from the joints | 2 | ||||
| Light‐headededness | 1 | Non‐pharmacological treatment | |||
| Cough | 1 | Stopped infusion | 43 | ||
| Headache | 1 | Lowered infusion rate | 4 | ||
| Increased swelling of the joints | 1 | ||||
| Increased intracranial pressure (feeling of) | 1 | ||||
| Muscular spasms | 1 |
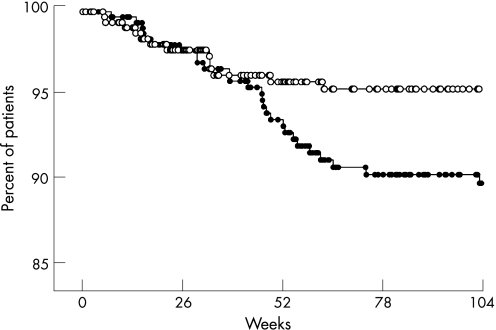
In the cohort, 323 out of 643 patients (50%; data missing for 30 patients) were on daily oral low‐dose glucocorticoids at baseline (median dose 5 mg/day). Fifteen of the 326 patients (4.6%) had an infusion reaction as compared with 28 out of 324 patients without glucocorticoid treatment (8.6%), p = 0.057. There was a 46.4% risk reduction (95% CI: 3.1 to 74.4%) by treatment with oral prednisolone, and dosage was not a significant predictor (not shown). Figure 3 shows a Kaplan–Meier plot illustrating the drug discontinuation rate due to treatment‐limiting infusion reactions caused by infliximab. Low‐dose glucocorticoids were associated with a significantly greater drug‐survival (p = 0.04) in a comparison by logrank test. Calculation indicated an NNT value of 25 (95% CI: 13 to 527) for oral prednisolone to prevent a treatment‐limiting infusion reaction during the study period. In the matched comparison, there was a significant difference in glucocorticoid use, as 15/43 (35%) of the cases were on low‐dose glucocorticoids compared with 28/43 (64%) of the controls (p = 0.017).
Figure 3 Drug discontinuation due to treatment‐limiting infusion reactions. Continuation rates are shown for patients treated with low‐dose glucocorticoids (white dots) as compared with patients not treated with low‐dose glucocorticoids (black dots), p = 0.04 by logrank test (Mantel–Cox).
Patients with infusion reactions had higher HAQ‐scores as compared with both the matched control and the cohort: the case group had a median value of 1.75 with an interquartile range of 1.13–2.25 vs 1.5 (0.63–2.25) for the matched control and 1.38 (0.88–1.88) for the cohort. There was also a non‐significant trend towards higher ESR in the cases compared with the cohort: 42.3±4.5 (mean±SEM) vs 34.4±1.1 (mean±SEM) (p = 0.066) (table 1). One additional risk factor for severe infusion reactions was identified in the matched comparison: the cases had failed to respond to treatment with a greater number of DMARDs than the matched controls (cases 3.65±0.32 (mean±SEM) vs controls 2.61±0.24 (mean±SEM), p = 0.012) before the start of treatment with infliximab (table 1).
MTX use was present in the vast majority of our patients. There was no difference in MTX use between the cases and the cohort. It was not possible to analyse this statistically further.
By a logistic regression done for continuous variables only, HAQ, DAS28, number of DMARDs and ESR were independent predictors of infusion reactions. The overall predictive power of these parameters, however, remained low.
Discussion
This retrospective study of a large patient cohort provides important information about the true incidence of treatment‐limiting infusion reactions and individual risk factors at baseline. We demonstrate a clear protective effect of daily low‐dose oral prednisolone which indicates a reduction in the risk for an infusion reaction by about half. The NNT for a 5‐year period to prevent a treatment‐limiting infusion reaction was calculated as 25 for low‐dose glucocorticoid treatment, which is on the same level as the summarised NNT of 26 for ramipril in the HOPE study.17 Patients with concurrent prednisolone had a significantly lower risk of experiencing a treatment‐limiting infusion reaction (as shown in fig. 3). In the matched comparison, there was a clear statistical difference in glucocorticoid use between the two groups, which further emphasises that glucocorticoids have a protective function. However, difference in glucocorticoid use did not affect the treatment duration within the case group itself, indicating that several risk factors interact. Hence, even though low‐dose glucocorticoid use has a protective effect against treatment‐limiting infusion reactions to infliximab, it does not affect the timing of the infusion reaction. Taken together, our results show that low‐dose glucocorticoids have several advantages for patients treated with infliximab. Many physicians tend to decrease or stop treatment with glucocorticoids, due to a fear of long‐term side effects, when the patient receives a positive response to DMARD treatment. However, low‐dose prednisolone has been shown to retard radiographic progression in patients with early RA, albeit with no significant difference in bone mineral density between the prednisolone and no‐prednisolone group over the 2‐year study period.18 The prophylactic effect of betamethasone given just prior to the infusion with infliximab was studied in a prospective manner by Sany et al.19 In that study, the occurrence of infusion reactions was lower in the placebo group, suggesting an increased risk with corticosteroid pretreatment, most likely explained by infusion reactions caused by the betamethasone itself. The use of oral steroids did not significantly affect the incidence of infusion reactions (14.3% with and 10.3% without oral corticosteroids, respectively, p = 0.28). However, this comparison did not correct for study treatment (betamethasone vs placebo), which did show a difference in use of oral corticosteroids between the placebo (84.7%) and betamethasone (77.1%) group. Thus, it appears that their results could well be consistent with ours. In a study by Wasserman et al,20 pretreatment with the antihistamine diphenhydramine did not reduce the frequency of infusion reactions. In this study, a rather wide definition of infusion reactions was used, and the conclusions might be biased by the known side effects of diphenhydramine.
The formation of anti‐infliximab antibodies, also known as human antichimeric antibodies, is a possible risk‐factor for the development of infusion reactions.21,22,23,24 A retrospective study by Haraoui et al25 found that RA patients who developed anti‐infliximab antibodies were about 10 years younger and were taking about half the mean dose of prednisone. In a previous study,6 it was found that the presence of anticardiolipin (aCL) antibodies in patients treated with infliximab was a risk factor for treatment‐limiting infusion reactions. Seventeen per cent of the aCL‐positive patients experienced such a reaction, compared with 5% of the patients in the whole registry.
A higher HAQ score and a higher number of previously failed DMARDs were also predictors of treatment‐limiting infusion reactions in this study. It is possible that the patients who experience a treatment‐limiting infusion reaction have suffered a higher inflammatory burden and therefore may be more prone to adverse events by various pharmacological agents, or that the chronic immunological activity itself may predispose to immunological adverse events. One possible confounder factor for this hypothesis is the decreased frequency of infusion reactions.
One important unexplained finding in this study was the sharply decreasing number of treatment limiting infusion reactions in our cohort. Several explanations could be considered. First, increased experience among physicians choosing the right pharmacological treatment for each patient. Second, the staff at the day care ward now has several years of experience and has learned to recognise the early signs of an infusion reaction, thus lowering the infusion rate before the reactions develop into something that might lead to discontinuation of treatment. Third, subtle changes in the manufacturing process of the drug may decrease the risk of immunological reactions.26 Our first assumption was that there could be an explanation in the fact that during the first years with TNF‐antagonists, there was a positive selection of patients with high disease activity, and this patient group could be more prone to have an adverse reaction. This seems not to be the case in our cohort, since the median DAS28‐scores are on the same level when making a yearly comparison over the study period (not shown). Similar results have been shown for etanercept by Feltelius et al.27
Conclusion
The use of daily low‐dose glucocorticoids is associated with a lower risk for treatment‐limiting infusion reactions to infliximab. Overall, treatment‐limiting infusion reactions have become significantly less common during the past 5 years.
Acknowledgements
The Swedish TNFα follow‐up registry, as a part of the Swedish ARTIS registry is owned by the Swedish Rheumatological Society (SRF), and supported by grants from Wyeth Lederle, Schering‐Plough and Abbott. This study was not supported financially by these companies and the companies were not involved in this study at any step.
Abbreviations
DAS - disease activity score
DMARD - disease‐modifying antirheumatic drug
ESR - erythrocyte sedimentation rate
HAQ - health‐assessment questionnaire
NNT - number needed to treat
RA - rheumatoid arthritis
TNF - tumour necrosis factor
References
- 1.Lipsky P E, van der Heijde D M, St Clair E W, Furst D E, Breedveld F C, Kalden J R.et al Infliximab and methotrexate in the treatment of rheumatoid arthritis. Anti‐Tumor Necrosis Factor Trial in Rheumatoid Arthritis with Concomitant Therapy Study Group. N Engl J Med 20003431594–1602. [DOI] [PubMed] [Google Scholar]
- 2.St Clair E W, van der Heijde D M, Smolen J S, Maini R N, Bathon J M, Emery P.et al Combination of infliximab and methotrexate therapy for early rheumatoid arthritis: a randomized, controlled trial. Arthritis Rheum 2004503432–3443. [DOI] [PubMed] [Google Scholar]
- 3.Goekoop‐Ruiterman Y P, de Vries‐Bouwstra J K, Allaart C F, van Zeben D, Kerstens P J, Hazes J M.et al Clinical and radiographic outcomes of four different treatment strategies in patients with early rheumatoid arthritis (the BeSt study): a randomized, controlled trial. Arthritis Rheum 2005523381–3390. [DOI] [PubMed] [Google Scholar]
- 4.van Vollenhoven R F, Klareskog L. Clinical responses to tumor necrosis factor alpha antagonists do not show a bimodal distribution: data from the Stockholm tumor necrosis factor alpha followup registry. Arthritis Rheum 2003481500–1503. [DOI] [PubMed] [Google Scholar]
- 5.Geborek P, Crnkic M, Petersson I F, Saxne T. Etanercept, infliximab, and leflunomide in established rheumatoid arthritis: clinical experience using a structured follow up programme in southern Sweden. Ann Rheum Dis 200261793–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jonsdottir T, Forslid J, van Vollenhoven A, Harju A, Brannemark S, Klareskog L.et al Treatment with tumour necrosis factor alpha antagonists in patients with rheumatoid arthritis induces anticardiolipin antibodies. Ann Rheum Dis 2004631075–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Flendrie M, Creemers M C, Welsing P M, den Broeder A A, van Riel P L. Survival during treatment with tumour necrosis factor blocking agents in rheumatoid arthritis. Ann Rheum Dis. 2003;62(Suppl II)30–33. [DOI] [PMC free article] [PubMed]
- 8.Gomez‐Reino J J, Carmona L, Valverde V R, Mola E M, Montero M D. Treatment of rheumatoid arthritis with tumor necrosis factor inhibitors may predispose to significant increase in tuberculosis risk: a multicenter active‐surveillance report. Arthritis Rheum 2003482122–2127. [DOI] [PubMed] [Google Scholar]
- 9.Carmona L, Gomez‐Reino J J, Rodriguez‐Valverde V, Montero D, Pascual‐Gomez E, Mola E M.et al Effectiveness of recommendations to prevent reactivation of latent tuberculosis infection in patients treated with tumor necrosis factor antagonists. Arthritis Rheum 2005521766–1772. [DOI] [PubMed] [Google Scholar]
- 10.Hyrich K, Symmons D, Watson K, Silman A. Baseline comorbidity levels in biologic and standard DMARD treated patients with rheumatoid arthritis: results from a national patient register. Ann Rheum Dis 200665895–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Franklin J, Lunt M, Bunn D, Symmons D, Silman A. Incidence of lymphoma in a large primary care derived cohort of cases of inflammatory polyarthritis. Ann Rheum Dis 200665617–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Crandall W V, Mackner L M. Infusion reactions to infliximab in children and adolescents: frequency, outcome and a predictive model. Aliment Pharmacol Ther 20031775–84. [DOI] [PubMed] [Google Scholar]
- 13.Kugathasan S, Levy M B, Saeian K, Vasilopoulos S, Kim J P, Prajapati D.et al Infliximab retreatment in adults and children with Crohn's disease: risk factors for the development of delayed severe systemic reaction. Am J Gastroenterol 2002971408–1414. [DOI] [PubMed] [Google Scholar]
- 14.van der Heijde D M, van't Hof M, van Riel P L, van de Putte L B. Development of a disease activity score based on judgment in clinical practice by rheumatologists. J Rheumatol 199320579–581. [PubMed] [Google Scholar]
- 15.Prevoo M L, van't Hof M A, Kuper H H, van Leeuwen M A, van de Putte L B, van Riel P L. Modified disease activity scores that include twenty‐eight‐joint counts. Development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis Rheum 19953844–48. [DOI] [PubMed] [Google Scholar]
- 16.McQuay H J, Moore R A. Using numerical results from systematic reviews in clinical practice. Ann Intern Med 1997126712–720. [DOI] [PubMed] [Google Scholar]
- 17.Smith M G, Neville A M, Middleton J C. Clinical and economic benefits of ramipril: an Australian analysis of the HOPE study. Intern Med J 200333414–419. [DOI] [PubMed] [Google Scholar]
- 18.Svensson B, Boonen A, Albertsson K, van der Heijde D, Keller C, Hafstrom I. Low‐dose prednisolone in addition to the initial disease‐modifying antirheumatic drug in patients with early active rheumatoid arthritis reduces joint destruction and increases the remission rate: a two‐year randomized trial. Arthritis Rheum 2005523360–3370. [DOI] [PubMed] [Google Scholar]
- 19.Sany J, Kaiser M J, Jorgensen C, Trape G. Study of the tolerance of infliximab infusions with or without betamethasone premedication in patients with active rheumatoid arthritis. Ann Rheum Dis 2005641647–1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wasserman M J, Weber D A, Guthrie J A, Bykerk V P, Lee P, Keystone E C. Infusion‐related reactions to infliximab in patients with rheumatoid arthritis in a clinical practice setting: relationship to dose, antihistamine pretreatment, and infusion number. J Rheumatol 2004311912–1917. [PubMed] [Google Scholar]
- 21.Baert F, Noman M, Vermeire S, Van Assche G, D'Haens G, Carbonez A.et al Influence of immunogenicity on the long‐term efficacy of infliximab in Crohn's disease. N Engl J Med 2003348601–608. [DOI] [PubMed] [Google Scholar]
- 22.Farrell R J, Alsahli M, Jeen Y T, Falchuk K R, Peppercorn M A, Michetti P. Intravenous hydrocortisone premedication reduces antibodies to infliximab in Crohn's disease: a randomized controlled trial. Gastroenterology 2003124917–924. [DOI] [PubMed] [Google Scholar]
- 23.Sandborn W J. Preventing antibodies to infliximab in patients with Crohn's disease: optimize not immunize. Gastroenterology 20031241140–1145. [DOI] [PubMed] [Google Scholar]
- 24.Maini R N, Breedveld F C, Kalden J R, Smolen J S, Davis D, Macfarlane J D.et al Therapeutic efficacy of multiple intravenous infusions of anti‐tumor necrosis factor alpha monoclonal antibody combined with low‐dose weekly methotrexate in rheumatoid arthritis. Arthritis Rheum 1998411552–1563. [DOI] [PubMed] [Google Scholar]
- 25.Haraoui B, Cameron L, Ouellet M, White B. Anti‐infliximab antibodies in patients with rheumatoid arthritis who require higher doses of infliximab to achieve or maintain a clinical response. J Rheumatol 20063331–36. [PubMed] [Google Scholar]
- 26. http://www.emea.europa.eu/humandocs/Humans/EPAR/remicade/remicade.htm 8b. Procedural steps taken and scientific information after cut‐off date (accessed 20 Dec 2006).
- 27.Feltelius N, Fored C M, Blomqvist P, Bertilsson L, Geborek P, Jacobsson L T.et al Results from a nationwide postmarketing cohort study of patients in Sweden treated with etanercept. Ann Rheum Dis 200564246–252. [DOI] [PMC free article] [PubMed] [Google Scholar]