Abstract
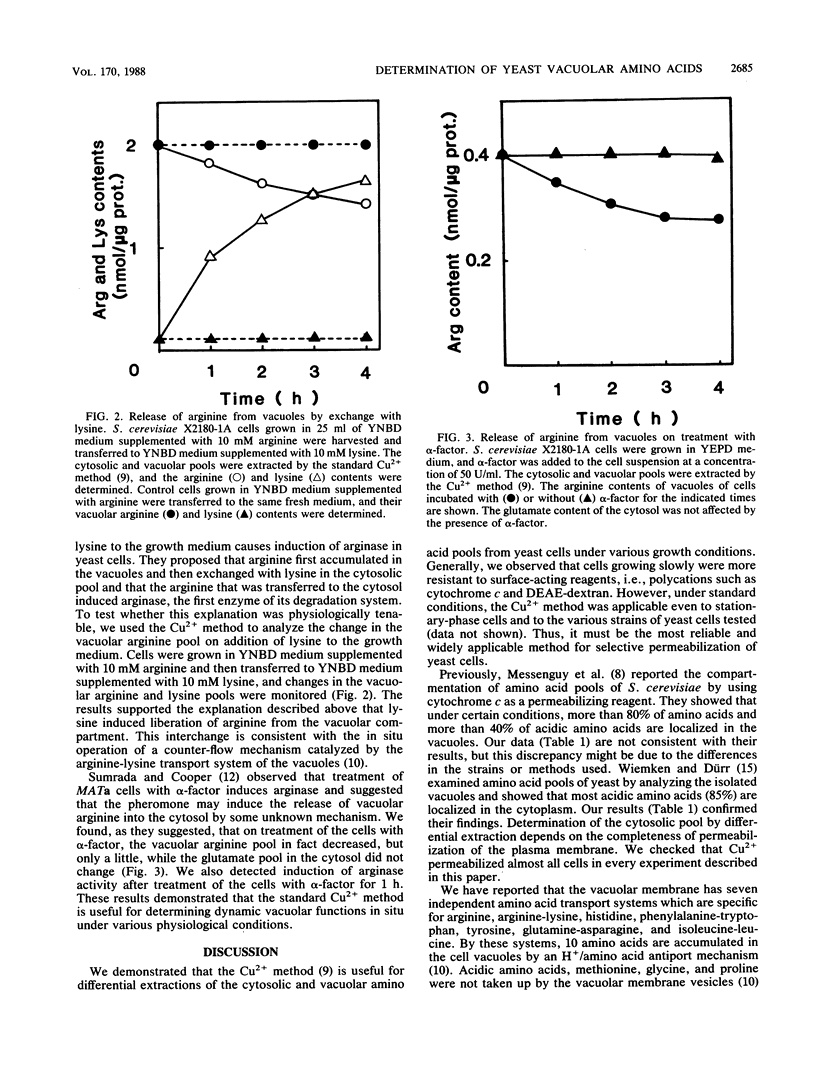
By using the Cu2+ method (Y. Ohsumi, K. Kitamoto, and Y. Anraku, J. Bacteriol. 170:2676-2682, 1988) for differential extraction of the vacuolar and cytosolic amino acid pools from yeast cells, the amino acid compositions of the two pools extracted from Saccharomyces cerevisiae cells, grown in synthetic medium supplemented with various amino acids, were determined. Histidine and lysine in the medium expanded the vacuolar pool extremely. Glutamate also accumulated in the cells, but mainly in the cytosol. The composition of amino acids in the cytosolic pool was fairly constant, in contrast to that in the vacuolar pool. Cells grown in synthetic medium supplemented with 10 mM arginine accumulated arginine in the vacuoles at a concentration of about 430 mM. This large arginine pool was metabolically active and was effectively utilized during nitrogen starvation. Arginine efflux from the vacuoles was coupled with K+ influx, with an arginine/K+ exchange ratio of 1, as judged by the initial rate. The vacuolar arginine pool was exchangeable with lysine added to the medium and was decreased by treatment of the cells with the mating pheromone, alpha-factor.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boller T., Dürr M., Wiemken A. Characterization of a specific transport system for arginine in isolated yeast vacuoles. Eur J Biochem. 1975 May;54(1):81–91. doi: 10.1111/j.1432-1033.1975.tb04116.x. [DOI] [PubMed] [Google Scholar]
- Crabeel M., Grenson M. Regulation of histidine uptake by specific feedback inhibition of two histidine permeases in Saccharomyces cerevisiae. Eur J Biochem. 1970 May 1;14(1):197–204. doi: 10.1111/j.1432-1033.1970.tb00278.x. [DOI] [PubMed] [Google Scholar]
- Duntze W., Stötzler D., Bücking-Throm E., Kalbitzer S. Purification and partial characterization of -factor, a mating-type specific inhibitor of cell reproduction from Saccharomyces cerevisiae. Eur J Biochem. 1973 Jun;35(2):357–365. doi: 10.1111/j.1432-1033.1973.tb02847.x. [DOI] [PubMed] [Google Scholar]
- Hennaut C., Hilger F., Grenson M. Space limitation for permease insertion in the cytoplasmic membrane of Saccharomyces cerevisiae. Biochem Biophys Res Commun. 1970 May 22;39(4):666–671. doi: 10.1016/0006-291x(70)90257-3. [DOI] [PubMed] [Google Scholar]
- Kitamoto K., Yoshizawa K., Ohsumi Y., Anraku Y. Mutants of Saccharomyces cerevisiae with defective vacuolar function. J Bacteriol. 1988 Jun;170(6):2687–2691. doi: 10.1128/jb.170.6.2687-2691.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messenguy F., Colin D., ten Have J. P. Regulation of compartmentation of amino acid pools in Saccharomyces cerevisiae and its effects on metabolic control. Eur J Biochem. 1980 Jul;108(2):439–447. doi: 10.1111/j.1432-1033.1980.tb04740.x. [DOI] [PubMed] [Google Scholar]
- Ohsumi Y., Kitamoto K., Anraku Y. Changes induced in the permeability barrier of the yeast plasma membrane by cupric ion. J Bacteriol. 1988 Jun;170(6):2676–2682. doi: 10.1128/jb.170.6.2676-2682.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato T., Ohsumi Y., Anraku Y. Substrate specificities of active transport systems for amino acids in vacuolar-membrane vesicles of Saccharomyces cerevisiae. Evidence of seven independent proton/amino acid antiport systems. J Biol Chem. 1984 Sep 25;259(18):11505–11508. [PubMed] [Google Scholar]
- Schwencke J., De Robichon-Szulmajster H. The transport of S-adenosyl-L-methionine in isolated yeast vacuoles and spheroplasts. Eur J Biochem. 1976 May 17;65(1):49–60. doi: 10.1111/j.1432-1033.1976.tb10388.x. [DOI] [PubMed] [Google Scholar]
- Sumrada R., Cooper T. G. Control of vacuole permeability and protein degradation by the cell cycle arrest signal in Saccharomyces cerevisiae. J Bacteriol. 1978 Oct;136(1):234–246. doi: 10.1128/jb.136.1.234-246.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson T. G. Amino-acid pool composition of Saccharomyces cerevisiae as a function of growth rate and amino-acid nitrogen source. J Gen Microbiol. 1976 Oct;96(2):263–268. doi: 10.1099/00221287-96-2-263. [DOI] [PubMed] [Google Scholar]
- Whitney P. A., Magasanik B. The induction of arginase in Saccharomyces cerevisiae. J Biol Chem. 1973 Sep 10;248(17):6197–6202. [PubMed] [Google Scholar]
- Wiemken A., Dürr M. Characterization of amino acid pools in the vacuolar compartment of Saccharomyces cerevisiae. Arch Microbiol. 1974;101(1):45–57. doi: 10.1007/BF00455924. [DOI] [PubMed] [Google Scholar]



