Abstract
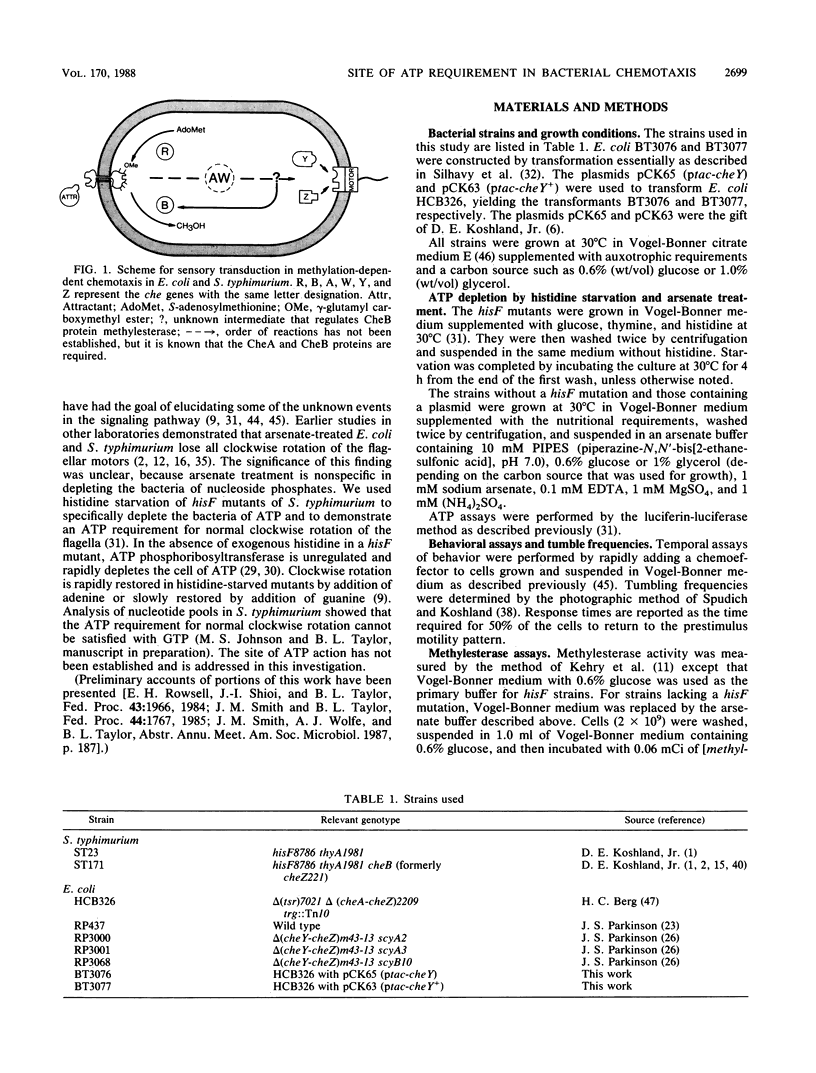
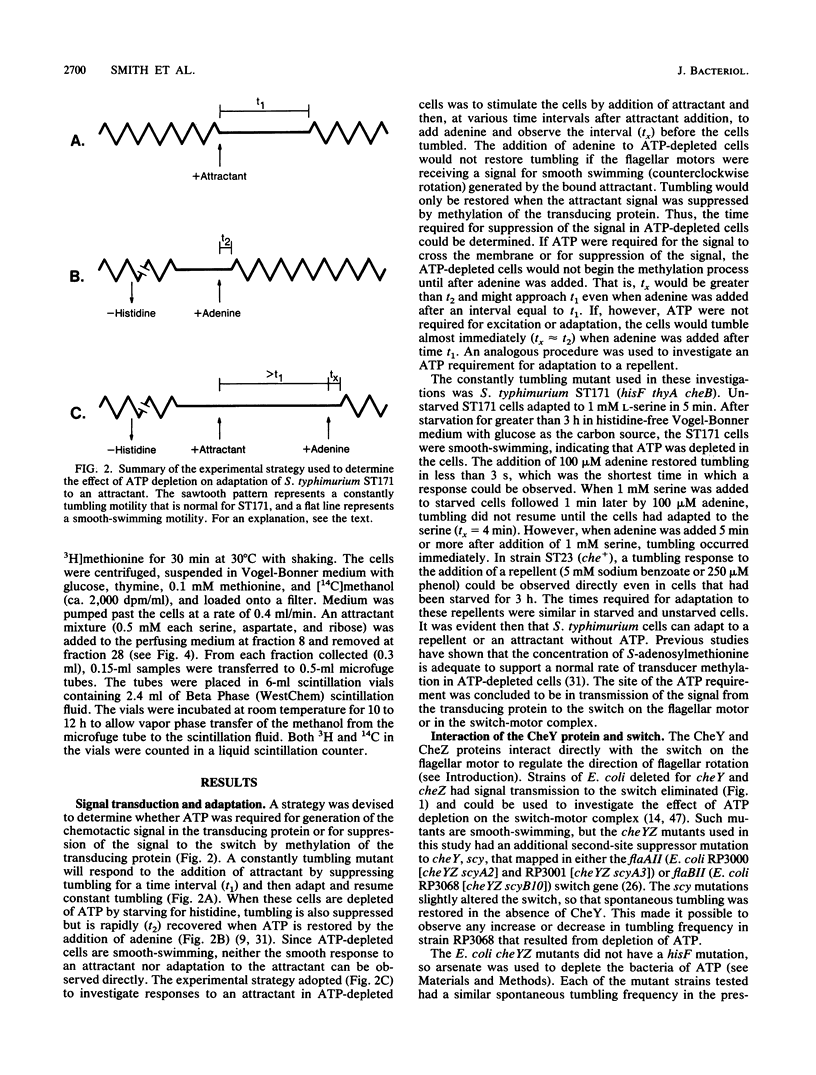
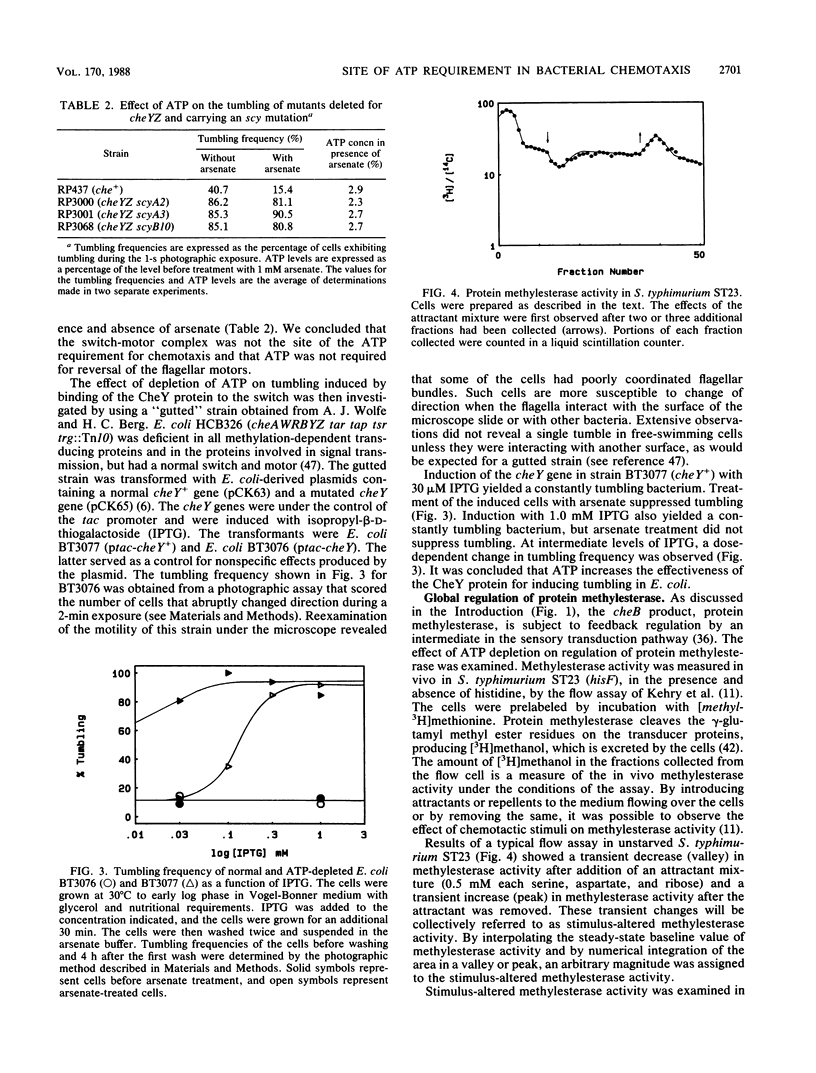
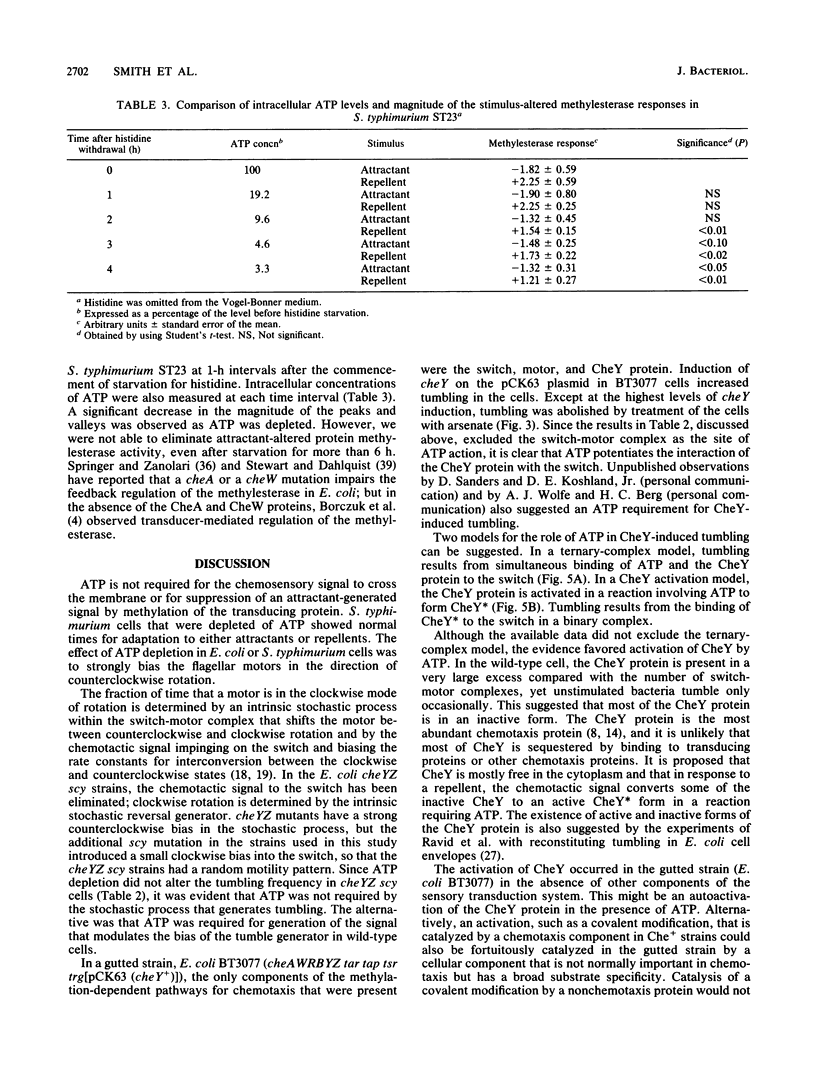
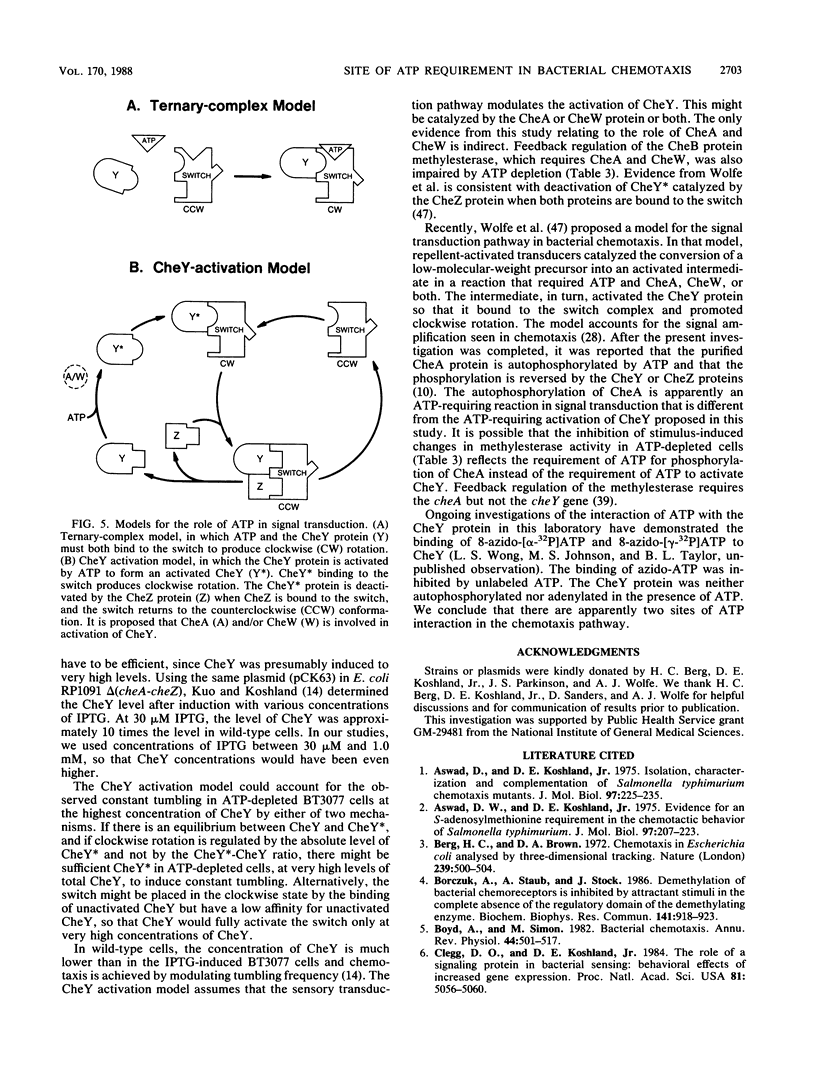
In Escherichia coli and Salmonella typhimurium, ATP is required for chemotaxis and for a normal probability of clockwise rotation of the flagellar motors, in addition to the requirement for S-adenosylmethionine (J. Shioi, R. J. Galloway, M. Niwano, R. E. Chinnock, and B. L. Taylor, J. Biol. Chem. 257:7969-7975, 1982). The site of the ATP requirement was investigated. The times required for S. typhimurium ST23 (hisF) to adapt to a step increase in serine, phenol, or benzoate were similar in cells depleted of ATP and in cells with normal levels of ATP. This established that ATP was not required for the chemotactic signal to cross the inner membrane or for adaptation to the transmembrane signal to occur. Depletion of ATP did not affect the probability of clockwise rotation in E. coli cheYZ scy strains that were defective in the cheY and cheZ genes and had a partially compensating mutation in the motor switch. Strain HCB326 (cheAWRBYZ tar tap tsr trg::Tn10), which was deficient in all chemotaxis components except the switch and motor, was transformed with the pCK63 plasmid (ptac-cheY+). Induction of cheY in the transformant increased the frequency of clockwise rotation, but except at the highest levels of CheY overproduction, clockwise rotation was abolished by depleting ATP. It is proposed that the CheY protein is normally in an inactive form and that ATP is required for formation of an active CheY* protein that binds to the switch on the flagellar motors and initiates clockwise rotation. Depletion of ATP partially inhibits feedback regulation of the cheB product, protein methylesterase, but this may reflect a second site of ATP action in chemotaxis.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aswad D. W., Koshland D. E., Jr Evidence for an S-adenosylmethionine requirement in the chemotactic behavior of Salmonella typhimurium. J Mol Biol. 1975 Sep 15;97(2):207–223. doi: 10.1016/s0022-2836(75)80035-0. [DOI] [PubMed] [Google Scholar]
- Aswad D., Koshland D. E., Jr Isolation, characterization and complementation of Salmonella typhimurium chemotaxis mutants. J Mol Biol. 1975 Sep 15;97(2):225–235. doi: 10.1016/s0022-2836(75)80036-2. [DOI] [PubMed] [Google Scholar]
- Berg H. C., Brown D. A. Chemotaxis in Escherichia coli analysed by three-dimensional tracking. Nature. 1972 Oct 27;239(5374):500–504. doi: 10.1038/239500a0. [DOI] [PubMed] [Google Scholar]
- Borczuk A., Staub A., Stock J. Demethylation of bacterial chemoreceptors is inhibited by attractant stimuli in the complete absence of the regulatory domain of the demethylating enzyme. Biochem Biophys Res Commun. 1986 Dec 30;141(3):918–923. doi: 10.1016/s0006-291x(86)80130-9. [DOI] [PubMed] [Google Scholar]
- Boyd A., Simon M. Bacterial chemotaxis. Annu Rev Physiol. 1982;44:501–517. doi: 10.1146/annurev.ph.44.030182.002441. [DOI] [PubMed] [Google Scholar]
- Clegg D. O., Koshland D. E., Jr The role of a signaling protein in bacterial sensing: behavioral effects of increased gene expression. Proc Natl Acad Sci U S A. 1984 Aug;81(16):5056–5060. doi: 10.1073/pnas.81.16.5056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang C. V., Niwano M., Ryu J., Taylor B. L. Inversion of aerotactic response in Escherichia coli deficient in cheB protein methylesterase. J Bacteriol. 1986 Apr;166(1):275–280. doi: 10.1128/jb.166.1.275-280.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFranco A. L., Koshland D. E., Jr Molecular cloning of chemotaxis genes and overproduction of gene products in the bacterial sensing system. J Bacteriol. 1981 Aug;147(2):390–400. doi: 10.1128/jb.147.2.390-400.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galloway R. J., Taylor B. L. Histidine starvation and adenosine 5'-triphosphate depletion in chemotaxis of Salmonella typhimurium. J Bacteriol. 1980 Dec;144(3):1068–1075. doi: 10.1128/jb.144.3.1068-1075.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess J. F., Oosawa K., Matsumura P., Simon M. I. Protein phosphorylation is involved in bacterial chemotaxis. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7609–7613. doi: 10.1073/pnas.84.21.7609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehry M. R., Doak T. G., Dahlquist F. W. Stimulus-induced changes in methylesterase activity during chemotaxis in Escherichia coli. J Biol Chem. 1984 Oct 10;259(19):11828–11835. [PubMed] [Google Scholar]
- Kondoh H. Tumbling chemotaxis mutants of Escherichia coli: possible gene-dependent effect of methionine starvation. J Bacteriol. 1980 May;142(2):527–534. doi: 10.1128/jb.142.2.527-534.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krikos A., Mutoh N., Boyd A., Simon M. I. Sensory transducers of E. coli are composed of discrete structural and functional domains. Cell. 1983 Jun;33(2):615–622. doi: 10.1016/0092-8674(83)90442-7. [DOI] [PubMed] [Google Scholar]
- Kuo S. C., Koshland D. E., Jr Roles of cheY and cheZ gene products in controlling flagellar rotation in bacterial chemotaxis of Escherichia coli. J Bacteriol. 1987 Mar;169(3):1307–1314. doi: 10.1128/jb.169.3.1307-1314.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutsukake K., Iino T. Refined genetic analysis of the region II che mutants in Salmonella typhimurium. Mol Gen Genet. 1985;199(3):406–409. doi: 10.1007/BF00330750. [DOI] [PubMed] [Google Scholar]
- Larsen S. H., Adler J., Gargus J. J., Hogg R. W. Chemomechanical coupling without ATP: the source of energy for motility and chemotaxis in bacteria. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1239–1243. doi: 10.1073/pnas.71.4.1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macnab R. M., Aizawa S. Bacterial motility and the bacterial flagellar motor. Annu Rev Biophys Bioeng. 1984;13:51–83. doi: 10.1146/annurev.bb.13.060184.000411. [DOI] [PubMed] [Google Scholar]
- Macnab R. M., Han D. P. Asynchronous switching of flagellar motors on a single bacterial cell. Cell. 1983 Jan;32(1):109–117. doi: 10.1016/0092-8674(83)90501-9. [DOI] [PubMed] [Google Scholar]
- Manson M. D., Blank V., Brade G., Higgins C. F. Peptide chemotaxis in E. coli involves the Tap signal transducer and the dipeptide permease. Nature. 1986 May 15;321(6067):253–256. doi: 10.1038/321253a0. [DOI] [PubMed] [Google Scholar]
- Niwano M., Taylor B. L. Novel sensory adaptation mechanism in bacterial chemotaxis to oxygen and phosphotransferase substrates. Proc Natl Acad Sci U S A. 1982 Jan;79(1):11–15. doi: 10.1073/pnas.79.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowlin D. M., Bollinger J., Hazelbauer G. L. Sites of covalent modification in Trg, a sensory transducer of Escherichia coli. J Biol Chem. 1987 May 5;262(13):6039–6045. [PubMed] [Google Scholar]
- Parkinson J. S. Complementation analysis and deletion mapping of Escherichia coli mutants defective in chemotaxis. J Bacteriol. 1978 Jul;135(1):45–53. doi: 10.1128/jb.135.1.45-53.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkinson J. S., Parker S. R., Talbert P. B., Houts S. E. Interactions between chemotaxis genes and flagellar genes in Escherichia coli. J Bacteriol. 1983 Jul;155(1):265–274. doi: 10.1128/jb.155.1.265-274.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravid S., Matsumura P., Eisenbach M. Restoration of flagellar clockwise rotation in bacterial envelopes by insertion of the chemotaxis protein CheY. Proc Natl Acad Sci U S A. 1986 Oct;83(19):7157–7161. doi: 10.1073/pnas.83.19.7157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHEDLOVSKY A. E., MAGASANIK B. A defect in histidine biosynthesis causing an adenine deficiency. J Biol Chem. 1962 Dec;237:3725–3730. [PubMed] [Google Scholar]
- SHEDLOVSKY A. E., MAGASANIK B. The enzymatic basis of an adenine-histidine relationship in Escherichia coli. J Biol Chem. 1962 Dec;237:3731–3736. [PubMed] [Google Scholar]
- Segall J. E., Block S. M., Berg H. C. Temporal comparisons in bacterial chemotaxis. Proc Natl Acad Sci U S A. 1986 Dec;83(23):8987–8991. doi: 10.1073/pnas.83.23.8987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shioi J. I., Galloway R. J., Niwano M., Chinnock R. E., Taylor B. L. Requirement of ATP in bacterial chemotaxis. J Biol Chem. 1982 Jul 25;257(14):7969–7975. [PubMed] [Google Scholar]
- Silverman M., Simon M. Flagellar rotation and the mechanism of bacterial motility. Nature. 1974 May 3;249(452):73–74. doi: 10.1038/249073a0. [DOI] [PubMed] [Google Scholar]
- Springer M. S., Goy M. F., Adler J. Protein methylation in behavioural control mechanisms and in signal transduction. Nature. 1979 Jul 26;280(5720):279–284. doi: 10.1038/280279a0. [DOI] [PubMed] [Google Scholar]
- Springer M. S., Kort E. N., Larsen S. H., Ordal G. W., Reader R. W., Adler J. Role of methionine in bacterial chemotaxis: requirement for tumbling and involvement in information processing. Proc Natl Acad Sci U S A. 1975 Nov;72(11):4640–4644. doi: 10.1073/pnas.72.11.4640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer M. S., Zanolari B. Sensory transduction in Escherichia coli: regulation of the demethylation rate by the CheA protein. Proc Natl Acad Sci U S A. 1984 Aug;81(16):5061–5065. doi: 10.1073/pnas.81.16.5061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer W. R., Koshland D. E., Jr Identification of a protein methyltransferase as the cheR gene product in the bacterial sensing system. Proc Natl Acad Sci U S A. 1977 Feb;74(2):533–537. doi: 10.1073/pnas.74.2.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spudich J. L., Koshland D. E., Jr Quantitation of the sensory response in bacterial chemotaxis. Proc Natl Acad Sci U S A. 1975 Feb;72(2):710–713. doi: 10.1073/pnas.72.2.710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stock A. M., Stock J. B. Purification and characterization of the CheZ protein of bacterial chemotaxis. J Bacteriol. 1987 Jul;169(7):3301–3311. doi: 10.1128/jb.169.7.3301-3311.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stock J. B., Koshland D. E., Jr A protein methylesterase involved in bacterial sensing. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3659–3663. doi: 10.1073/pnas.75.8.3659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor B. L. Role of proton motive force in sensory transduction in bacteria. Annu Rev Microbiol. 1983;37:551–573. doi: 10.1146/annurev.mi.37.100183.003003. [DOI] [PubMed] [Google Scholar]
- Tribhuwan R. C., Johnson M. S., Taylor B. L. Evidence against direct involvement of cyclic GMP or cyclic AMP in bacterial chemotactic signaling. J Bacteriol. 1986 Nov;168(2):624–630. doi: 10.1128/jb.168.2.624-630.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VOGEL H. J., BONNER D. M. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956 Jan;218(1):97–106. [PubMed] [Google Scholar]
- Wolfe A. J., Conley M. P., Kramer T. J., Berg H. C. Reconstitution of signaling in bacterial chemotaxis. J Bacteriol. 1987 May;169(5):1878–1885. doi: 10.1128/jb.169.5.1878-1885.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]



