Abstract
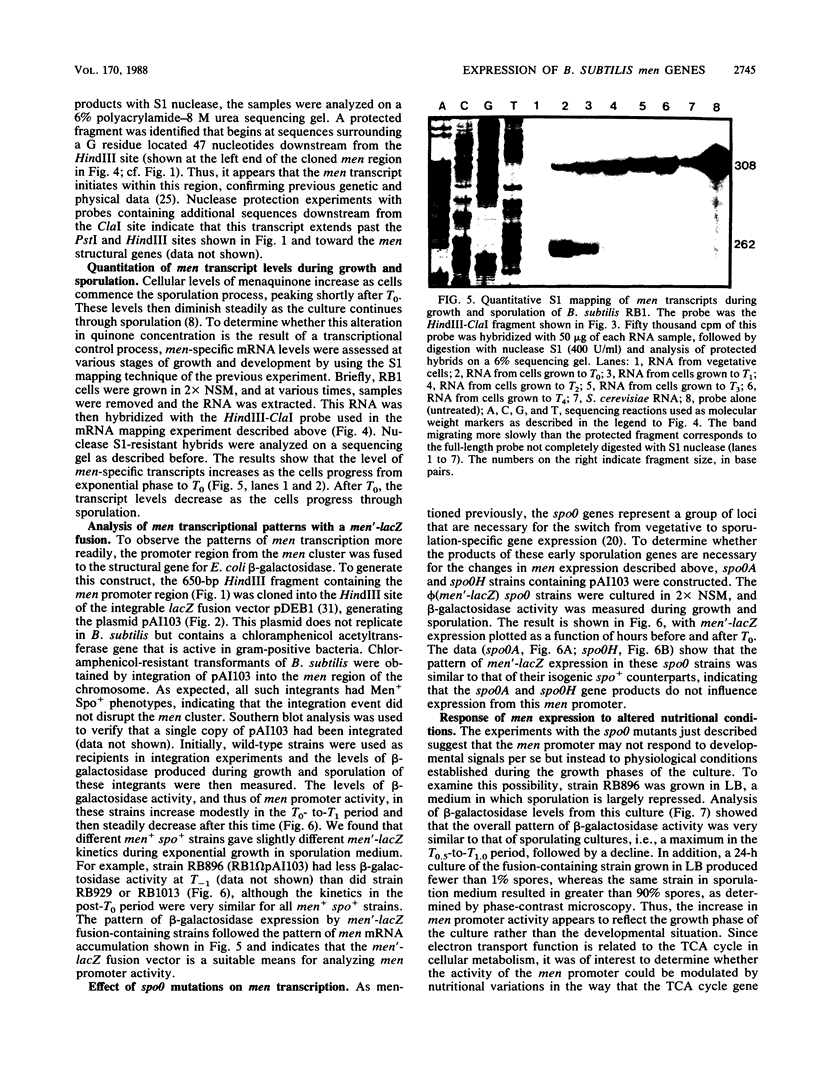
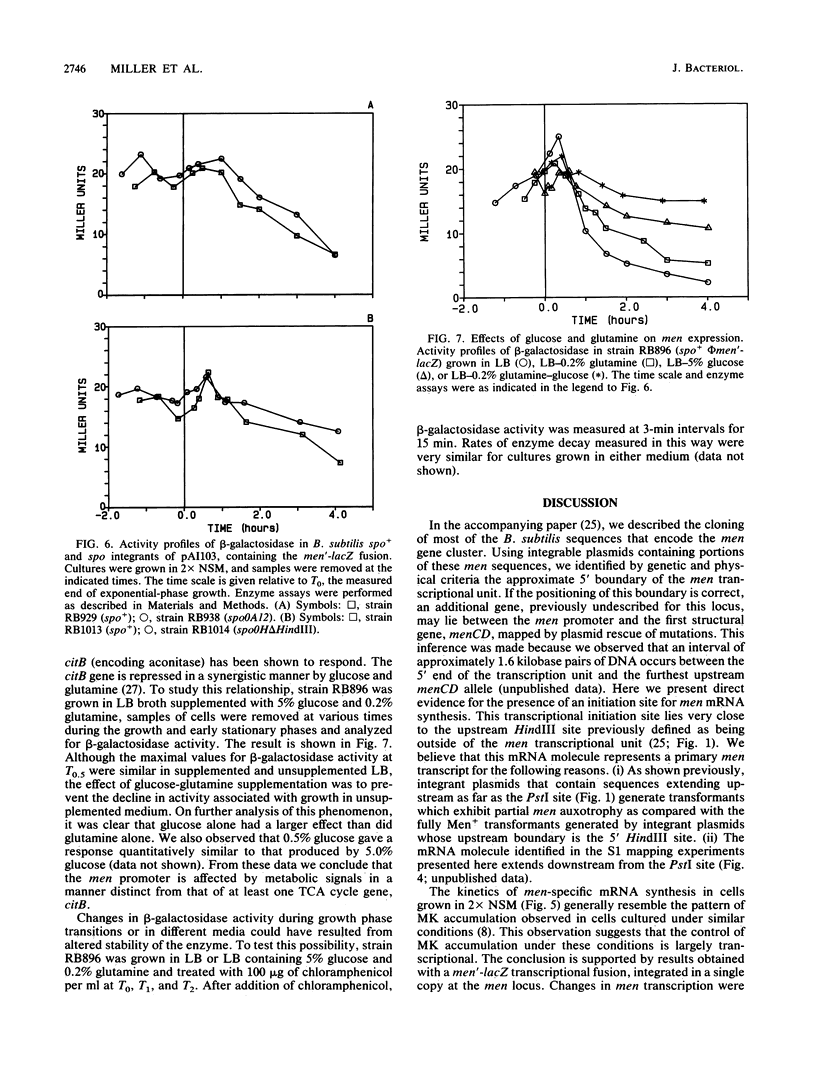
The control of men gene expression during growth and sporulation of Bacillus subtilis was examined at the transcriptional level. Two different approaches were used. (i) Steady-state levels of men-specific mRNA were measured directly. (ii) A men'-lacZ gene fusion was constructed. In both cases, it was observed that men promoter activity was maximal at the onset of sporulation and declined soon thereafter. These kinetics were similar to the pattern of menaquinone accumulation previously observed. Expression from the men promoter was independent of the presence of the products of the spo0A and spo0H genes and was enhanced by addition of glucose and glutamine to the culture medium. DNA sequence analysis of the promoter region revealed a potential recognition site for the principal vegetative form of RNA polymerase but not for any of the known minor polymerase forms. The functionality in vivo of the promoter sequence was confirmed by high-resolution S1 nuclease mapping of the transcript start site. An additional sequence element was identified that is shared by the sdhA, citG, and ctaA promoters and may indicate a common regulatory mechanism in the expression of these genes.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CHAIX P., PETIT J. F. Influence du taux de croissance sur la constitution du spectre hématinique de B. subtilis. Biochim Biophys Acta. 1957 Sep;25(3):481–486. doi: 10.1016/0006-3002(57)90517-6. [DOI] [PubMed] [Google Scholar]
- Chen E. Y., Seeburg P. H. Supercoil sequencing: a fast and simple method for sequencing plasmid DNA. DNA. 1985 Apr;4(2):165–170. doi: 10.1089/dna.1985.4.165. [DOI] [PubMed] [Google Scholar]
- Dingman D. W., Rosenkrantz M. S., Sonenshein A. L. Relationship between aconitase gene expression and sporulation in Bacillus subtilis. J Bacteriol. 1987 Jul;169(7):3068–3075. doi: 10.1128/jb.169.7.3068-3075.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doi R. H., Wang L. F. Multiple procaryotic ribonucleic acid polymerase sigma factors. Microbiol Rev. 1986 Sep;50(3):227–243. doi: 10.1128/mr.50.3.227-243.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrand S. K., Taber H. W. Changes in menaquinone concentration during growth and early sporulation in Bacillus subtilis. J Bacteriol. 1974 Jan;117(1):324–326. doi: 10.1128/jb.117.1.324-326.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrand S. K., Taber H. W. Physiological effects of menaquinone deficiency in Bacillus subtilis. J Bacteriol. 1973 Sep;115(3):1035–1044. doi: 10.1128/jb.115.3.1035-1044.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favaloro J., Treisman R., Kamen R. Transcription maps of polyoma virus-specific RNA: analysis by two-dimensional nuclease S1 gel mapping. Methods Enzymol. 1980;65(1):718–749. doi: 10.1016/s0076-6879(80)65070-8. [DOI] [PubMed] [Google Scholar]
- Fortnagel P. The regulation of aconitase and isocitrate dehydrogenase in sporulation mutants of Bacillus subtilis. Biochim Biophys Acta. 1970 Nov 24;222(2):290–298. doi: 10.1016/0304-4165(70)90116-9. [DOI] [PubMed] [Google Scholar]
- Graves M. C., Rabinowitz J. C. In vivo and in vitro transcription of the Clostridium pasteurianum ferredoxin gene. Evidence for "extended" promoter elements in gram-positive organisms. J Biol Chem. 1986 Aug 25;261(24):11409–11415. [PubMed] [Google Scholar]
- HANSON H. M., WITOSLAWSKI J. J., CAMPBELL E. H. REVERSIBLE DISRUPTION OF A WAVELENGTH DISCRIMINATION IN PIGEONS FOLLOWING ADMINISTRATION OF PHENIPRAZINE. Toxicol Appl Pharmacol. 1964 Nov;6:690–695. doi: 10.1016/0041-008x(64)90119-x. [DOI] [PubMed] [Google Scholar]
- Hanson R. S., Cox D. P. Effect of different nutritional conditions on the synthesis of tricarboxylic acid cycle enzymes. J Bacteriol. 1967 Jun;93(6):1777–1787. doi: 10.1128/jb.93.6.1777-1787.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawley D. K., McClure W. R. Compilation and analysis of Escherichia coli promoter DNA sequences. Nucleic Acids Res. 1983 Apr 25;11(8):2237–2255. doi: 10.1093/nar/11.8.2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill D. E., Hope I. A., Macke J. P., Struhl K. Saturation mutagenesis of the yeast his3 regulatory site: requirements for transcriptional induction and for binding by GCN4 activator protein. Science. 1986 Oct 24;234(4775):451–457. doi: 10.1126/science.3532321. [DOI] [PubMed] [Google Scholar]
- Igo M. M., Losick R. Regulation of a promoter that is utilized by minor forms of RNA polymerase holoenzyme in Bacillus subtilis. J Mol Biol. 1986 Oct 20;191(4):615–624. doi: 10.1016/0022-2836(86)90449-3. [DOI] [PubMed] [Google Scholar]
- Kudo T., Yoshitake J., Kato C., Usami R., Horikoshi K. Cloning of a developmentally regulated element from alkalophilic Bacillus subtilis DNA. J Bacteriol. 1985 Jan;161(1):158–163. doi: 10.1128/jb.161.1.158-163.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Losick R., Youngman P., Piggot P. J. Genetics of endospore formation in Bacillus subtilis. Annu Rev Genet. 1986;20:625–669. doi: 10.1146/annurev.ge.20.120186.003205. [DOI] [PubMed] [Google Scholar]
- Magnusson K., Philips M. K., Guest J. R., Rutberg L. Nucleotide sequence of the gene for cytochrome b558 of the Bacillus subtilis succinate dehydrogenase complex. J Bacteriol. 1986 Jun;166(3):1067–1071. doi: 10.1128/jb.166.3.1067-1071.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messing J. New M13 vectors for cloning. Methods Enzymol. 1983;101:20–78. doi: 10.1016/0076-6879(83)01005-8. [DOI] [PubMed] [Google Scholar]
- Miles J. S., Guest J. R. Complete nucleotide sequence of the fumarase gene (citG) of Bacillus subtilis 168. Nucleic Acids Res. 1985 Jan 11;13(1):131–140. doi: 10.1093/nar/13.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller P., Rabinowitz A., Taber H. Molecular cloning and preliminary genetic analysis of the men gene cluster of Bacillus subtilis. J Bacteriol. 1988 Jun;170(6):2735–2741. doi: 10.1128/jb.170.6.2735-2741.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran C. P., Jr, Lang N., LeGrice S. F., Lee G., Stephens M., Sonenshein A. L., Pero J., Losick R. Nucleotide sequences that signal the initiation of transcription and translation in Bacillus subtilis. Mol Gen Genet. 1982;186(3):339–346. doi: 10.1007/BF00729452. [DOI] [PubMed] [Google Scholar]
- Rosenkrantz M. S., Dingman D. W., Sonenshein A. L. Bacillus subtilis citB gene is regulated synergistically by glucose and glutamine. J Bacteriol. 1985 Oct;164(1):155–164. doi: 10.1128/jb.164.1.155-164.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders C. W., Schmidt B. J., Mirot M. S., Thompson L. D., Guyer M. S. Use of chromosomal integration in the establishment and expression of blaZ, a Staphylococcus aureus beta-lactamase gene, in Bacillus subtilis. J Bacteriol. 1984 Mar;157(3):718–726. doi: 10.1128/jb.157.3.718-726.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaeffer P., Millet J., Aubert J. P. Catabolic repression of bacterial sporulation. Proc Natl Acad Sci U S A. 1965 Sep;54(3):704–711. doi: 10.1073/pnas.54.3.704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreier H. J., Sonenshein A. L. Altered regulation of the glnA gene in glutamine synthetase mutants of Bacillus subtilis. J Bacteriol. 1986 Jul;167(1):35–43. doi: 10.1128/jb.167.1.35-43.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith M. C., Mountain A., Baumberg S. Sequence analysis of the Bacillus subtilis argC promoter region. Gene. 1986;49(1):53–60. doi: 10.1016/0378-1119(86)90384-7. [DOI] [PubMed] [Google Scholar]
- Zalkin H., Ebbole D. J. Organization and regulation of genes encoding biosynthetic enzymes in Bacillus subtilis. J Biol Chem. 1988 Feb 5;263(4):1595–1598. [PubMed] [Google Scholar]
- Zuber P., Losick R. Use of a lacZ fusion to study the role of the spoO genes of Bacillus subtilis in developmental regulation. Cell. 1983 Nov;35(1):275–283. doi: 10.1016/0092-8674(83)90230-1. [DOI] [PubMed] [Google Scholar]