Abstract
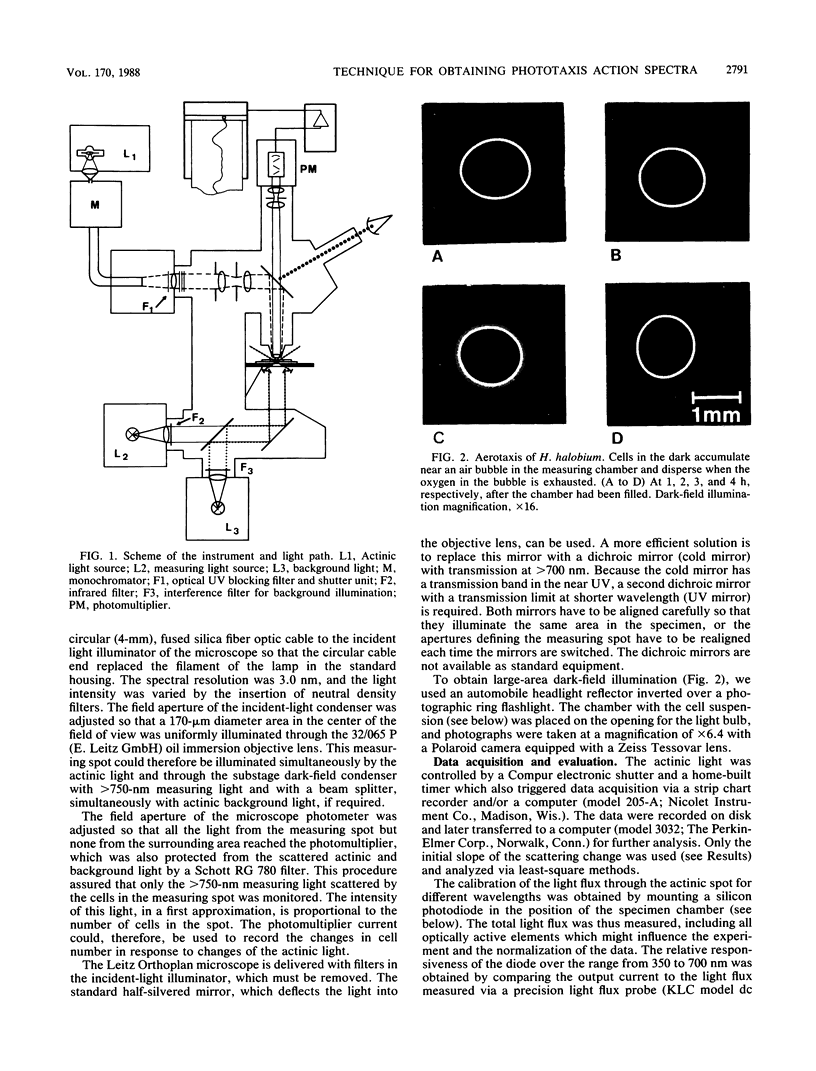
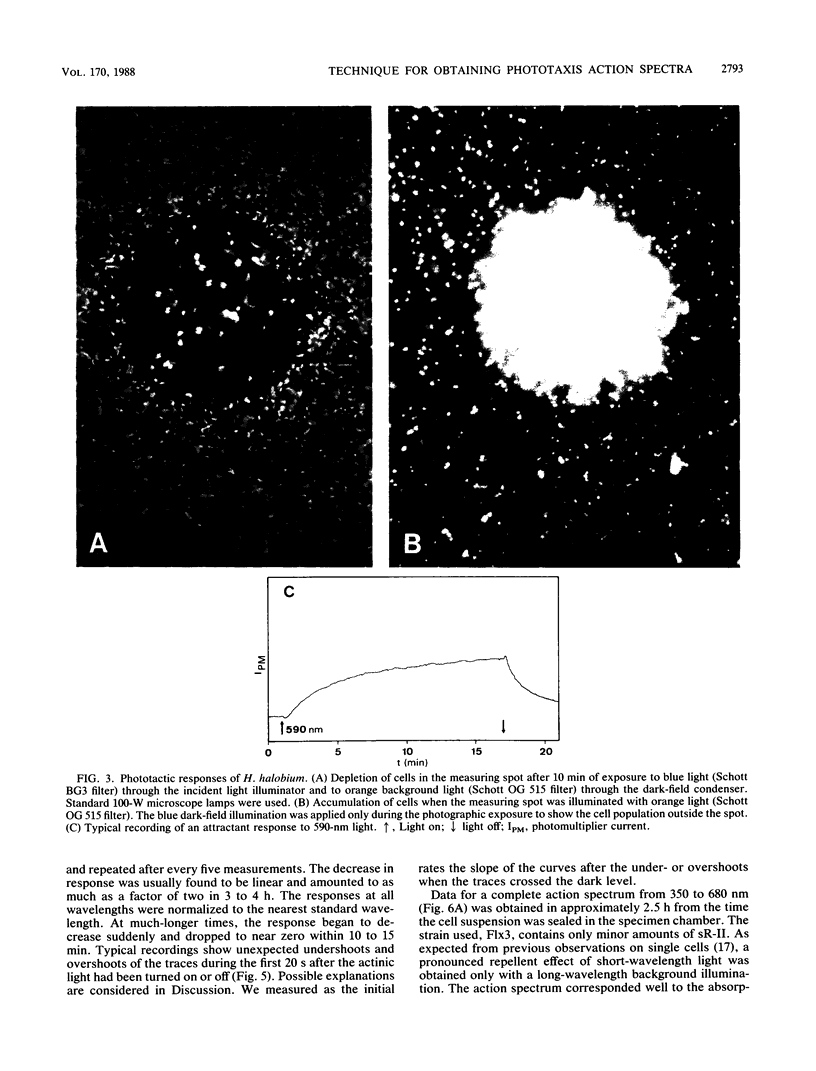
We have developed a simple and rapid technique for measuring the action spectra for phototaxis of populations of microorganisms and applied it to halobacteria. A microscope with a dark-field condenser was used to illuminate the cell suspension in a sealed chamber with light of wavelength greater than 750 nm; in this region of the spectrum, the halobacteria show no phototactic response. A 150-micron spot of light from a xenon arc lamp, whose wavelength and intensity can be varied, was projected through the objective lens into the center of the dark field. The objective lens imaged this measuring spot through a 780-nm cut-off filter on an aperture in front of a photomultiplier. The intensity of the scattered 750-nm light, and therefore the photomultiplier current, is proportional to the number of cells in the measuring spot. A third lamp provided background light of variable wavelength and intensity through the dark-field condenser. To minimize secondary effects due to large changes in cell density, we recorded the initial changes in the photomultiplier current over 1 min after the actinic light had been switched on. By plotting the rate of change against wavelength, we obtained action spectra after the proper corrections for changes in light intensity with wavelength were applied and saturation effects were avoided.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dencher N. A., Hildebrand E. Sensory transduction in Halobacterium halobium: retinal protein pigment controls UV-induced behavioral response. Z Naturforsch C. 1979 Sep-Oct;34(9-10):841–847. doi: 10.1515/znc-1979-9-1030. [DOI] [PubMed] [Google Scholar]
- Diehn B. Action spectra of the phototactic responses in Euglena. Biochim Biophys Acta. 1969 Feb 18;177(1):136–143. doi: 10.1016/0304-4165(69)90073-7. [DOI] [PubMed] [Google Scholar]
- Dundas I. E. Physiology of halobacteriaceae. Adv Microb Physiol. 1977;15:85–120. doi: 10.1016/s0065-2911(08)60315-x. [DOI] [PubMed] [Google Scholar]
- Hildebrand E., Schimz A. Integration of photosensory signals in Halobacterium halobium. J Bacteriol. 1986 Jul;167(1):305–311. doi: 10.1128/jb.167.1.305-311.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildebrand E., Schimz A. Role of the response oscillator in inverse responses of Halobacterium halobium to weak light stimuli. J Bacteriol. 1987 Jan;169(1):254–259. doi: 10.1128/jb.169.1.254-259.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Häder D. P., Lebert M. Real time computer-controlled tracking of motile microorganisms. Photochem Photobiol. 1985 Nov;42(5):509–514. doi: 10.1111/j.1751-1097.1985.tb01602.x. [DOI] [PubMed] [Google Scholar]
- Marwan W., Oesterhelt D. Signal formation in the halobacterial photophobic response mediated by a fourth retinal protein (P480). J Mol Biol. 1987 May 20;195(2):333–342. doi: 10.1016/0022-2836(87)90654-1. [DOI] [PubMed] [Google Scholar]
- McCain D. A., Amici L. A., Spudich J. L. Kinetically resolved states of the Halobacterium halobium flagellar motor switch and modulation of the switch by sensory rhodopsin I. J Bacteriol. 1987 Oct;169(10):4750–4758. doi: 10.1128/jb.169.10.4750-4758.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spudich E. N., Spudich J. L. Control of transmembrane ion fluxes to select halorhodopsin-deficient and other energy-transduction mutants of Halobacterium halobium. Proc Natl Acad Sci U S A. 1982 Jul;79(14):4308–4312. doi: 10.1073/pnas.79.14.4308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spudich J. L., Bogomolni R. A. Mechanism of colour discrimination by a bacterial sensory rhodopsin. Nature. 1984 Dec 6;312(5994):509–513. doi: 10.1038/312509a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spudich J. L., Bogomolni R. A. Spectroscopic discrimination of the three rhodopsinlike pigments in Halobacterium halobium membranes. Biophys J. 1983 Aug;43(2):243–246. doi: 10.1016/S0006-3495(83)84345-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoeckenius W. The rhodopsin-like pigments of halobacteria: light-energy and signal transducers in an archaebacterium. Trends Biochem Sci. 1985 Dec;10(12):483–486. doi: 10.1016/0968-0004(85)90210-5. [DOI] [PubMed] [Google Scholar]
- Sundberg S. A., Bogomolni R. A., Spudich J. L. Selection and properties of phototaxis-deficient mutants of Halobacterium halobium. J Bacteriol. 1985 Oct;164(1):282–287. doi: 10.1128/jb.164.1.282-287.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi T., Watanabe M., Kamo N., Kobatake Y. Negative phototaxis from blue light and the role of third rhodopsinlike pigment in halobacterium cutirubrum. Biophys J. 1985 Aug;48(2):235–240. doi: 10.1016/S0006-3495(85)83776-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff E. K., Bogomolni R. A., Scherrer P., Hess B., Stoeckenius W. Color discrimination in halobacteria: spectroscopic characterization of a second sensory receptor covering the blue-green region of the spectrum. Proc Natl Acad Sci U S A. 1986 Oct;83(19):7272–7276. doi: 10.1073/pnas.83.19.7272. [DOI] [PMC free article] [PubMed] [Google Scholar]