Abstract
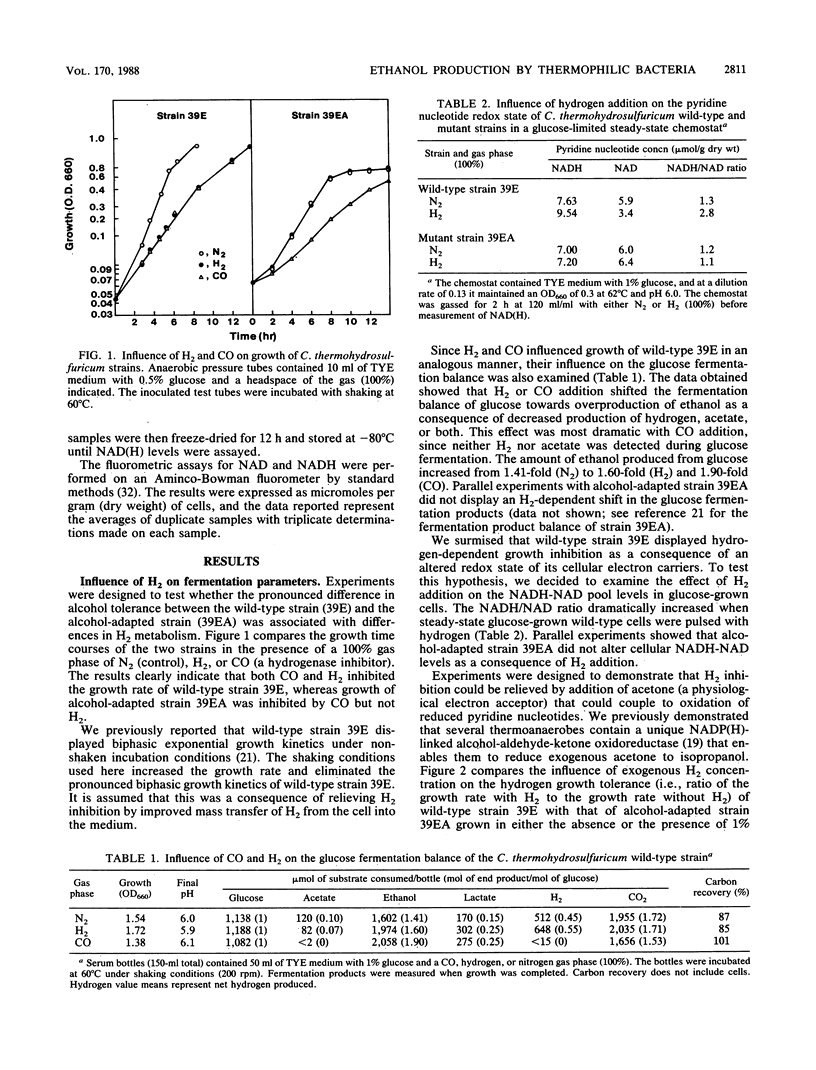
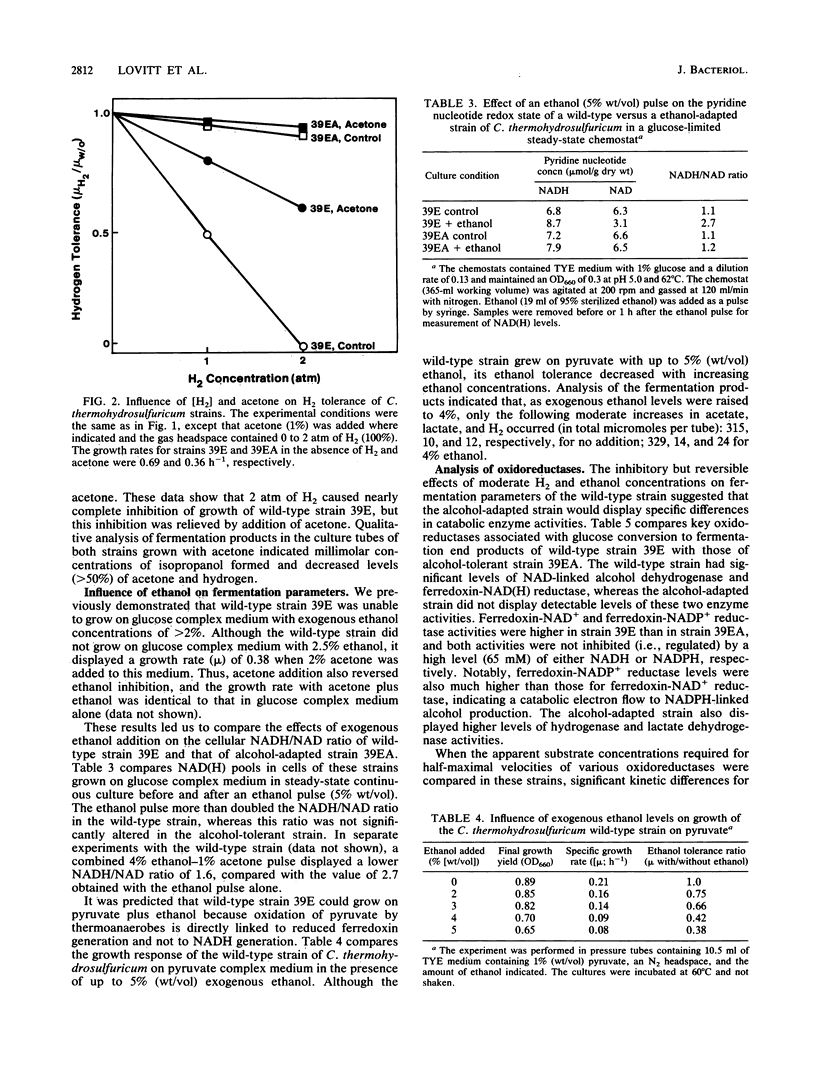
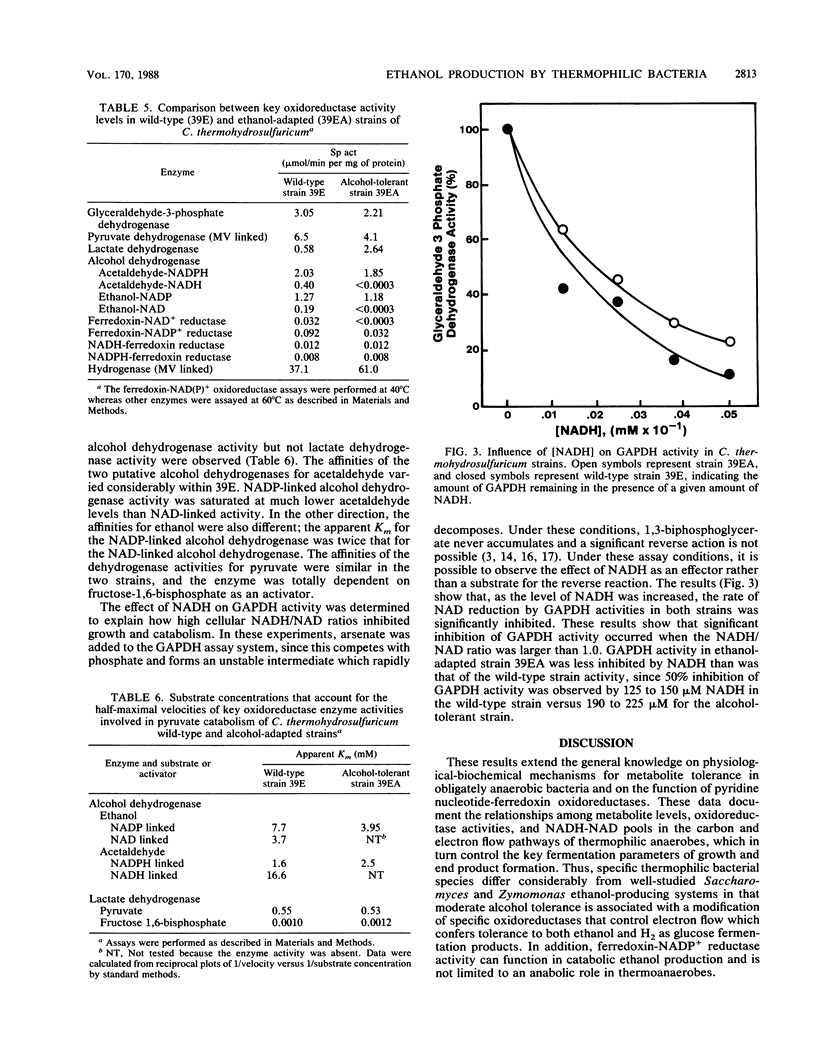
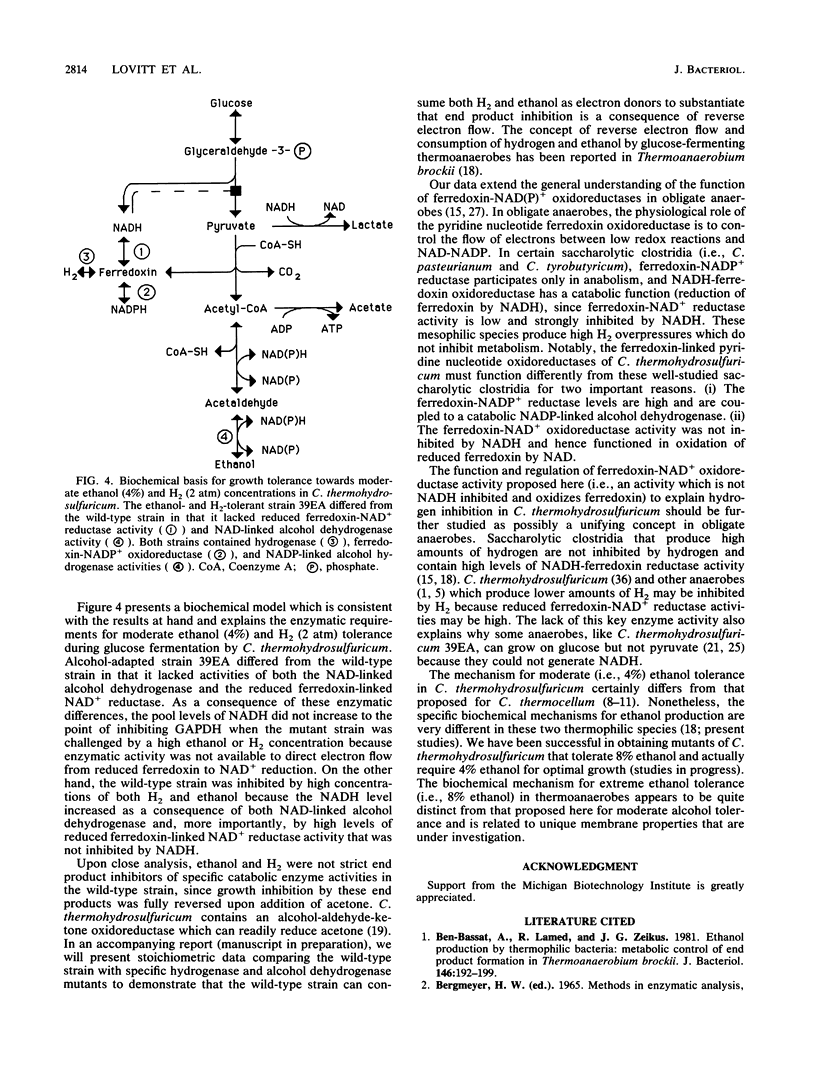
The metabolic and enzymatic bases for growth tolerance to ethanol (4%) and H2 (2 atm [1 atm = 101.29 kPa]) fermentation products in Clostridium thermohydrosulfuricum were compared in a sensitive wild-type strain and an insensitive alcohol-adapted strain. In the wild-type strain, ethanol (4%) and H2 (2 atm) inhibited glucose but not pyruvate fermentation parameters (growth and end product formation). Inhibition of glucose fermentation by ethanol (4%) in the wild-type strain was reversed by addition of acetone (1%), which lowered H2 and ethanol production while increasing isopropanol and acetate production. Pulsing cells grown in continuous culture on glucose with 5% ethanol or 1 atm of H2 significantly raised the NADH/NAD ratio in the wild-type strain but not in the alcohol-adapted strain. Analysis of key oxidoreductases demonstrated that the alcohol-adapted strain lacked detectable levels of reduced ferredoxin-linked NAD reductase and NAD-linked alcohol dehydrogenase activities which were present in the wild-type strain. Differences in the glucose fermentation product ratios of the two strains were related to differences in lactate dehydrogenase and hydrogenase levels and sensitivity of glyceraldehyde 3-phosphate dehydrogenase activity to NADH inhibition. A biochemical model is proposed which describes a common enzymatic mechanism for growth tolerance of thermoanaerobes to moderate concentrations of both ethanol and hydrogen.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ben-Bassat A., Lamed R., Zeikus J. G. Ethanol production by thermophilic bacteria: metabolic control of end product formation in Thermoanaerobium brockii. J Bacteriol. 1981 Apr;146(1):192–199. doi: 10.1128/jb.146.1.192-199.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blusson H., Petitdemange H., Gay R. A new, fast, and sensitive assay for NADH--ferredoxin oxidoreductase detection in clostridia. Anal Biochem. 1981 Jan 1;110(1):176–181. doi: 10.1016/0003-2697(81)90132-9. [DOI] [PubMed] [Google Scholar]
- Byers L. D. Glyceraldehyde-3-phosphate dehydrogenase from yeast. Methods Enzymol. 1982;89(Pt 500):326–335. doi: 10.1016/s0076-6879(82)89059-9. [DOI] [PubMed] [Google Scholar]
- Chung K. T. Inhibitory effects of H2 on growth of Clostridium cellobioparum. Appl Environ Microbiol. 1976 Mar;31(3):342–348. doi: 10.1128/aem.31.3.342-348.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garvie E. I. Bacterial lactate dehydrogenases. Microbiol Rev. 1980 Mar;44(1):106–139. doi: 10.1128/mr.44.1.106-139.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrero A. A., Gomez R. F. Development of ethanol tolerance in Clostridium thermocellum: effect of growth temperature. Appl Environ Microbiol. 1980 Sep;40(3):571–577. doi: 10.1128/aem.40.3.571-577.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrero A. A., Gomez R. F., Roberts M. F. Ethanol-induced changes in the membrane lipid composition of Clostridium thermocellum. Biochim Biophys Acta. 1982 Dec 8;693(1):195–204. doi: 10.1016/0005-2736(82)90487-4. [DOI] [PubMed] [Google Scholar]
- Hyun H. H., Shen G. J., Zeikus J. G. Differential amylosaccharide metabolism of Clostridium thermosulfurogenes and Clostridium thermohydrosulfuricum. J Bacteriol. 1985 Dec;164(3):1153–1161. doi: 10.1128/jb.164.3.1153-1161.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyun H. H., Zeikus J. G., Longin R., Millet J., Ryter A. Ultrastructure and extreme heat resistance of spores from thermophilic Clostridium species. J Bacteriol. 1983 Dec;156(3):1332–1337. doi: 10.1128/jb.156.3.1332-1337.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyun H. H., Zeikus J. G. Simultaneous and Enhanced Production of Thermostable Amylases and Ethanol from Starch by Cocultures of Clostridium thermosulfurogenes and Clostridium thermohydrosulfuricum. Appl Environ Microbiol. 1985 May;49(5):1174–1181. doi: 10.1128/aem.49.5.1174-1181.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jungermann K., Thauer R. K., Leimenstoll G., Decker K. Function of reduced pyridine nucleotide-ferredoxin oxidoreductases in saccharolytic Clostridia. Biochim Biophys Acta. 1973 May 30;305(2):268–280. doi: 10.1016/0005-2728(73)90175-8. [DOI] [PubMed] [Google Scholar]
- Krebs H. A., Freedland R. A., Hems R., Stubbs M. Inhibition of hepatic gluconeogenesis by ethanol. Biochem J. 1969 Mar;112(1):117–124. doi: 10.1042/bj1120117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., PASSONNEAU J. V., ROCK M. K. The stability of pyridine nucleotides. J Biol Chem. 1961 Oct;236:2756–2759. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lamed R. J., Zeikus J. G. Novel NADP-linked alcohol--aldehyde/ketone oxidoreductase in thermophilic ethanologenic bacteria. Biochem J. 1981 Apr 1;195(1):183–190. doi: 10.1042/bj1950183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamed R., Zeikus J. G. Ethanol production by thermophilic bacteria: relationship between fermentation product yields of and catabolic enzyme activities in Clostridium thermocellum and Thermoanaerobium brockii. J Bacteriol. 1980 Nov;144(2):569–578. doi: 10.1128/jb.144.2.569-578.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ljungdahl L. G., Bryant F., Carreira L., Saiki T., Wiegel J. Some aspects of thermophilic and extreme thermophilic anaerobic microorganisms. Basic Life Sci. 1981;18:397–419. doi: 10.1007/978-1-4684-3980-9_23. [DOI] [PubMed] [Google Scholar]
- Lovitt R. W., Longin R., Zeikus J. G. Ethanol Production by Thermophilic Bacteria: Physiological Comparison of Solvent Effects on Parent and Alcohol-Tolerant Strains of Clostridium thermohydrosulfuricum. Appl Environ Microbiol. 1984 Jul;48(1):171–177. doi: 10.1128/aem.48.1.171-177.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynd L., Kerby R., Zeikus J. G. Carbon monoxide metabolism of the methylotrophic acidogen Butyribacterium methylotrophicum. J Bacteriol. 1982 Jan;149(1):255–263. doi: 10.1128/jb.149.1.255-263.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng T. K., Ben-Bassat A., Zeikus J. G. Ethanol Production by Thermophilic Bacteria: Fermentation of Cellulosic Substrates by Cocultures of Clostridium thermocellum and Clostridium thermohydrosulfuricum. Appl Environ Microbiol. 1981 Jun;41(6):1337–1343. doi: 10.1128/aem.41.6.1337-1343.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petitdemange H., Cherrier C., Raval R., Gay R. Regulation of the NADH and NADPH-ferredoxin oxidoreductases in clostridia of the butyric group. Biochim Biophys Acta. 1976 Feb 24;421(2):334–337. doi: 10.1016/0304-4165(76)90300-7. [DOI] [PubMed] [Google Scholar]
- WOLIN M. J. FRUCTOSE-1,6-DIPHOSPHATE REQUIREMENT OF STREPTOCOCCAL LACTIC DEHYDROGENASES. Science. 1964 Nov 6;146(3645):775–777. doi: 10.1126/science.146.3645.775. [DOI] [PubMed] [Google Scholar]
- Wiegel J., Ljungdahl L. G., Rawson J. R. Isolation from soil and properties of the extreme thermophile Clostridium thermohydrosulfuricum. J Bacteriol. 1979 Sep;139(3):800–810. doi: 10.1128/jb.139.3.800-810.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeikus J. G., Ben-Bassat A., Hegge P. W. Microbiology of methanogenesis in thermal, volcanic environments. J Bacteriol. 1980 Jul;143(1):432–440. doi: 10.1128/jb.143.1.432-440.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeikus J. G., Ben-Bassat A., Ng T. K., Lamed R. J. Thermophilic ethanol fermentations. Basic Life Sci. 1981;18:441–461. doi: 10.1007/978-1-4684-3980-9_26. [DOI] [PubMed] [Google Scholar]
- Zeikus J. G., Fuchs G., Kenealy W., Thauer R. K. Oxidoreductases involved in cell carbon synthesis of Methanobacterium thermoautotrophicum. J Bacteriol. 1977 Nov;132(2):604–613. doi: 10.1128/jb.132.2.604-613.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]


