Abstract
We show that several interacting environmental factors influence the topology of intracellular DNA. Negative supercoiling of DNA in vivo is increased by anaerobic growth and is also influenced by growth phase. The tonB promoter of Escherichia coli and Salmonella typhimurium was found to be highly sensitive to changes in DNA supercoiling. Expression was increased by novobiocin, an inhibitor of DNA gyrase, and was decreased by factors which increase DNA superhelicity. Expression of the plasmid-encoded tonB gene was enhanced by gamma delta insertions in cis in a distance- and orientation-independent fashion. Both the res site and the TnpR protein of gamma delta, which is known to function as a type I topoisomerase, were required for this activation. tonB expression increased during the growth cycle and was reduced by anaerobiosis. There was excellent correlation between tonB expression from a plasmid and the level of supercoiling of that plasmid under a wide range of conditions. The chromosomal tonB gene was regulated in a manner identical to that of the plasmid-encoded gene. Thus, the physiological regulation of tonB expression in response to anaerobiosis and growth phase appears to be mediated by environmentally induced changes in DNA superhelicity.
Full text
PDF


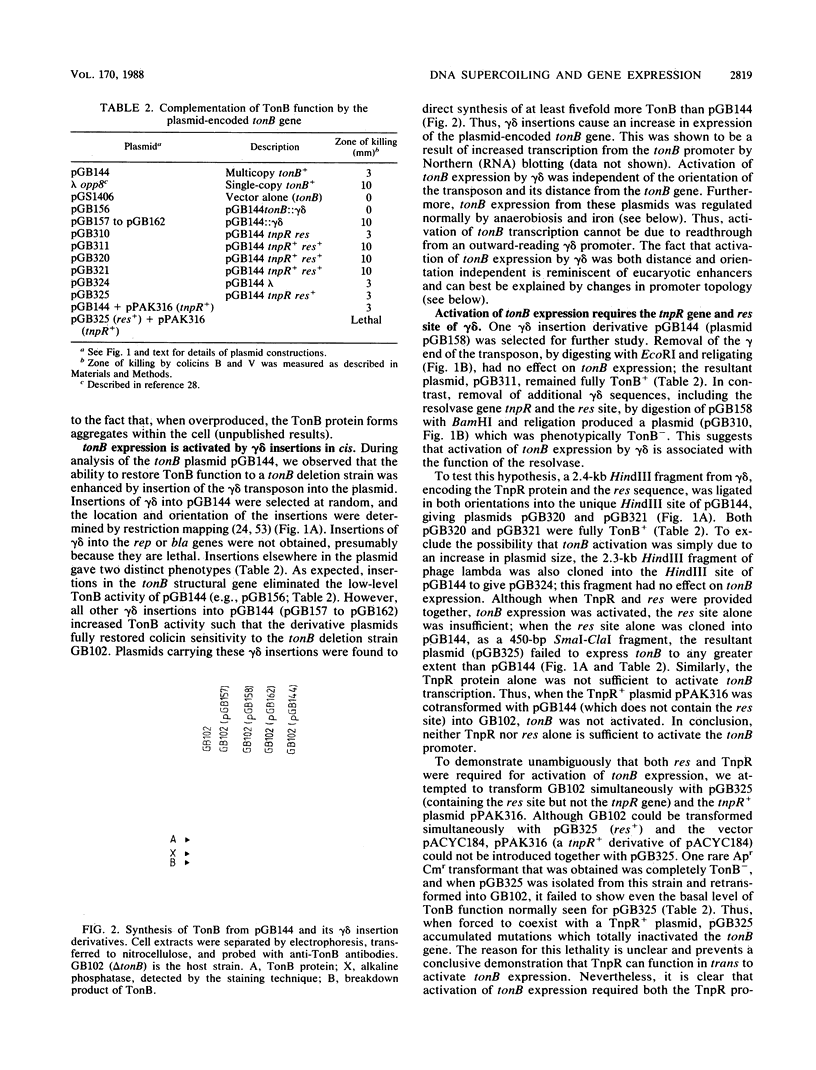

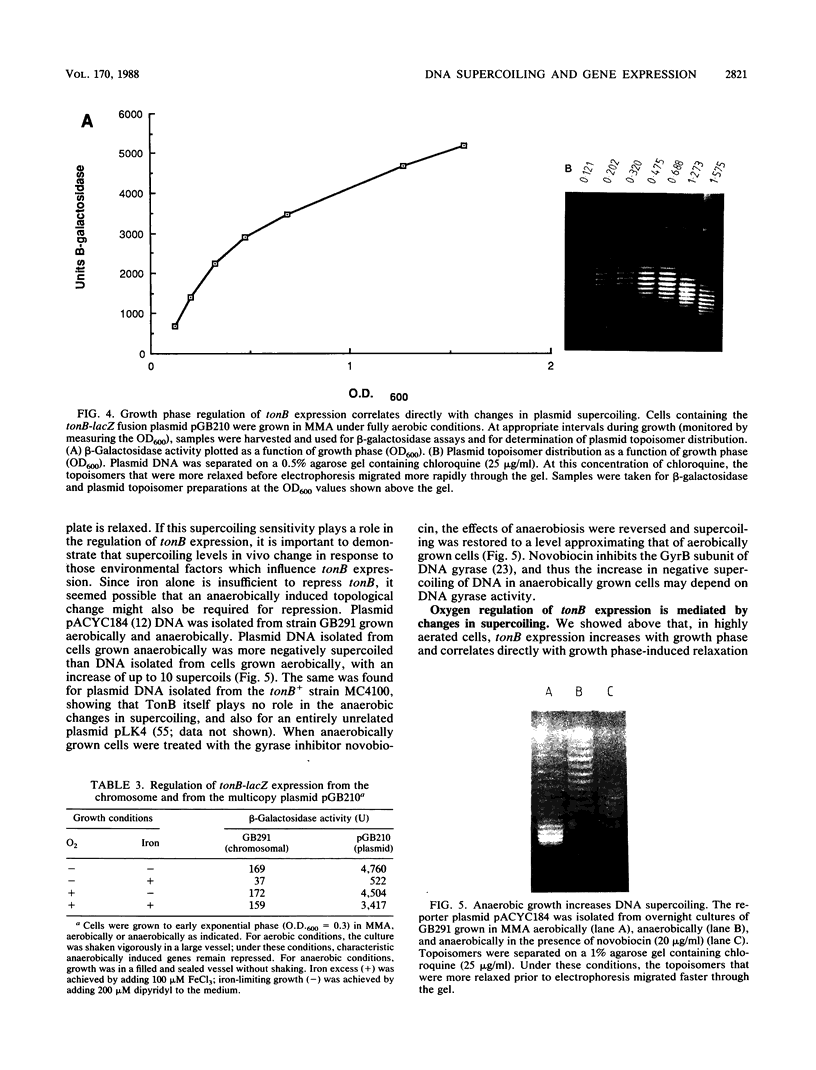





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aliabadi Z., Warren F., Mya S., Foster J. W. Oxygen-regulated stimulons of Salmonella typhimurium identified by Mu d(Ap lac) operon fusions. J Bacteriol. 1986 Mar;165(3):780–786. doi: 10.1128/jb.165.3.780-786.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ames G. F. Resolution of bacterial proteins by polyacrylamide gel electrophoresis on slabs. Membrane, soluble, and periplasmic fractions. J Biol Chem. 1974 Jan 25;249(2):634–644. [PubMed] [Google Scholar]
- Bagg A., Neilands J. B. Ferric uptake regulation protein acts as a repressor, employing iron (II) as a cofactor to bind the operator of an iron transport operon in Escherichia coli. Biochemistry. 1987 Aug 25;26(17):5471–5477. doi: 10.1021/bi00391a039. [DOI] [PubMed] [Google Scholar]
- Balke V. L., Gralla J. D. Changes in the linking number of supercoiled DNA accompany growth transitions in Escherichia coli. J Bacteriol. 1987 Oct;169(10):4499–4506. doi: 10.1128/jb.169.10.4499-4506.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett E. L., Kwan H. S., Macy J. Anaerobiosis, formate, nitrate, and pyrA are involved in the regulation of formate hydrogenlyase in Salmonella typhimurium. J Bacteriol. 1984 Jun;158(3):972–977. doi: 10.1128/jb.158.3.972-977.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman M. L., Jackson D. E. Selection of lac gene fusions in vivo: ompR-lacZ fusions that define a functional domain of the ompR gene product. J Bacteriol. 1984 Aug;159(2):750–756. doi: 10.1128/jb.159.2.750-756.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliska J. B., Cozzarelli N. R. Use of site-specific recombination as a probe of DNA structure and metabolism in vivo. J Mol Biol. 1987 Mar 20;194(2):205–218. doi: 10.1016/0022-2836(87)90369-x. [DOI] [PubMed] [Google Scholar]
- Borowiec J. A., Gralla J. D. All three elements of the lac ps promoter mediate its transcriptional response to DNA supercoiling. J Mol Biol. 1987 May 5;195(1):89–97. doi: 10.1016/0022-2836(87)90329-9. [DOI] [PubMed] [Google Scholar]
- Bremer E., Silhavy T. J., Weinstock G. M. Transposable lambda placMu bacteriophages for creating lacZ operon fusions and kanamycin resistance insertions in Escherichia coli. J Bacteriol. 1985 Jun;162(3):1092–1099. doi: 10.1128/jb.162.3.1092-1099.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casadaban M. J., Chou J., Cohen S. N. In vitro gene fusions that join an enzymatically active beta-galactosidase segment to amino-terminal fragments of exogenous proteins: Escherichia coli plasmid vectors for the detection and cloning of translational initiation signals. J Bacteriol. 1980 Aug;143(2):971–980. doi: 10.1128/jb.143.2.971-980.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casadaban M. J. Transposition and fusion of the lac genes to selected promoters in Escherichia coli using bacteriophage lambda and Mu. J Mol Biol. 1976 Jul 5;104(3):541–555. doi: 10.1016/0022-2836(76)90119-4. [DOI] [PubMed] [Google Scholar]
- Chang A. C., Cohen S. N. Construction and characterization of amplifiable multicopy DNA cloning vehicles derived from the P15A cryptic miniplasmid. J Bacteriol. 1978 Jun;134(3):1141–1156. doi: 10.1128/jb.134.3.1141-1156.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman D. C., Foster T. J. Analysis of the reduction in expression of tetracycline resistance determined by transposon Tn10 in the multicopy state. Mol Gen Genet. 1981;182(1):171–177. doi: 10.1007/BF00422786. [DOI] [PubMed] [Google Scholar]
- Connell N., Han Z., Moreno F., Kolter R. An E. coli promoter induced by the cessation of growth. Mol Microbiol. 1987 Sep;1(2):195–201. doi: 10.1111/j.1365-2958.1987.tb00512.x. [DOI] [PubMed] [Google Scholar]
- DiNardo S., Voelkel K. A., Sternglanz R., Reynolds A. E., Wright A. Escherichia coli DNA topoisomerase I mutants have compensatory mutations in DNA gyrase genes. Cell. 1982 Nov;31(1):43–51. doi: 10.1016/0092-8674(82)90403-2. [DOI] [PubMed] [Google Scholar]
- Drlica K. Biology of bacterial deoxyribonucleic acid topoisomerases. Microbiol Rev. 1984 Dec;48(4):273–289. doi: 10.1128/mr.48.4.273-289.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drlica K., Rouviere-Yaniv J. Histonelike proteins of bacteria. Microbiol Rev. 1987 Sep;51(3):301–319. doi: 10.1128/mr.51.3.301-319.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellison M. J., Fenton M. J., Ho P. S., Rich A. Long-range interactions of multiple DNA structural transitions within a common topological domain. EMBO J. 1987 May;6(5):1513–1522. doi: 10.1002/j.1460-2075.1987.tb02394.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans J. S., Levine B. A., Trayer I. P., Dorman C. J., Higgins C. F. Sequence-imposed structural constraints in the TonB protein of E. coli. FEBS Lett. 1986 Nov 24;208(2):211–216. doi: 10.1016/0014-5793(86)81020-1. [DOI] [PubMed] [Google Scholar]
- Fleming T. P., Nahlik M. S., McIntosh M. A. Regulation of enterobactin iron transport in Escherichia coli: characterization of ent::Mu d(Apr lac) operon fusions. J Bacteriol. 1983 Dec;156(3):1171–1177. doi: 10.1128/jb.156.3.1171-1177.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gellert M., O'Dea M. H., Itoh T., Tomizawa J. Novobiocin and coumermycin inhibit DNA supercoiling catalyzed by DNA gyrase. Proc Natl Acad Sci U S A. 1976 Dec;73(12):4474–4478. doi: 10.1073/pnas.73.12.4474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyer M. S. The gamma delta sequence of F is an insertion sequence. J Mol Biol. 1978 Dec 15;126(3):347–365. doi: 10.1016/0022-2836(78)90045-1. [DOI] [PubMed] [Google Scholar]
- Hantke K. Regulation of ferric iron transport in Escherichia coli K12: isolation of a constitutive mutant. Mol Gen Genet. 1981;182(2):288–292. doi: 10.1007/BF00269672. [DOI] [PubMed] [Google Scholar]
- Hatfull G. F., Noble S. M., Grindley N. D. The gamma delta resolvase induces an unusual DNA structure at the recombinational crossover point. Cell. 1987 Apr 10;49(1):103–110. doi: 10.1016/0092-8674(87)90760-4. [DOI] [PubMed] [Google Scholar]
- Heffron F., McCarthy B. J., Ohtsubo H., Ohtsubo E. DNA sequence analysis of the transposon Tn3: three genes and three sites involved in transposition of Tn3. Cell. 1979 Dec;18(4):1153–1163. doi: 10.1016/0092-8674(79)90228-9. [DOI] [PubMed] [Google Scholar]
- Higgins C. F., Dorman C. J., Stirling D. A., Waddell L., Booth I. R., May G., Bremer E. A physiological role for DNA supercoiling in the osmotic regulation of gene expression in S. typhimurium and E. coli. Cell. 1988 Feb 26;52(4):569–584. doi: 10.1016/0092-8674(88)90470-9. [DOI] [PubMed] [Google Scholar]
- Jamieson D. J., Higgins C. F. Anaerobic and leucine-dependent expression of a peptide transport gene in Salmonella typhimurium. J Bacteriol. 1984 Oct;160(1):131–136. doi: 10.1128/jb.160.1.131-136.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamieson D. J., Higgins C. F. Two genetically distinct pathways for transcriptional regulation of anaerobic gene expression in Salmonella typhimurium. J Bacteriol. 1986 Oct;168(1):389–397. doi: 10.1128/jb.168.1.389-397.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayaraman P. S., Peakman T. C., Busby S. J., Quincey R. V., Cole J. A. Location and sequence of the promoter of the gene for the NADH-dependent nitrite reductase of Escherichia coli and its regulation by oxygen, the Fnr protein and nitrite. J Mol Biol. 1987 Aug 20;196(4):781–788. doi: 10.1016/0022-2836(87)90404-9. [DOI] [PubMed] [Google Scholar]
- Jovanovich S. B., Lebowitz J. Estimation of the effect of coumermycin A1 on Salmonella typhimurium promoters by using random operon fusions. J Bacteriol. 1987 Oct;169(10):4431–4435. doi: 10.1128/jb.169.10.4431-4435.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knecht D. A., Dimond R. L. Visualization of antigenic proteins on Western blots. Anal Biochem. 1984 Jan;136(1):180–184. doi: 10.1016/0003-2697(84)90321-x. [DOI] [PubMed] [Google Scholar]
- Kranz R. G., Haselkorn R. Anaerobic regulation of nitrogen-fixation genes in Rhodopseudomonas capsulata. Proc Natl Acad Sci U S A. 1986 Sep;83(18):6805–6809. doi: 10.1073/pnas.83.18.6805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krasnow M. A., Cozzarelli N. R. Site-specific relaxation and recombination by the Tn3 resolvase: recognition of the DNA path between oriented res sites. Cell. 1983 Apr;32(4):1313–1324. doi: 10.1016/0092-8674(83)90312-4. [DOI] [PubMed] [Google Scholar]
- Kuritzkes D. R., Zhang X. Y., Lin E. C. Use of phi(glp-lac) in studies of respiratory regulation of the Escherichia coli anaerobic sn-glycerol-3-phosphate dehydrogenase genes (glpAB). J Bacteriol. 1984 Feb;157(2):591–598. doi: 10.1128/jb.157.2.591-598.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lambden P. R., Guest J. R. Mutants of Escherichia coli K12 unable to use fumarate as an anaerobic electron acceptor. J Gen Microbiol. 1976 Dec;97(2):145–160. doi: 10.1099/00221287-97-2-145. [DOI] [PubMed] [Google Scholar]
- Liu L. F., Wang J. C. Supercoiling of the DNA template during transcription. Proc Natl Acad Sci U S A. 1987 Oct;84(20):7024–7027. doi: 10.1073/pnas.84.20.7024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menzel R., Gellert M. Fusions of the Escherichia coli gyrA and gyrB control regions to the galactokinase gene are inducible by coumermycin treatment. J Bacteriol. 1987 Mar;169(3):1272–1278. doi: 10.1128/jb.169.3.1272-1278.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menzel R., Gellert M. Regulation of the genes for E. coli DNA gyrase: homeostatic control of DNA supercoiling. Cell. 1983 Aug;34(1):105–113. doi: 10.1016/0092-8674(83)90140-x. [DOI] [PubMed] [Google Scholar]
- Neilands J. B. Microbial envelope proteins related to iron. Annu Rev Microbiol. 1982;36:285–309. doi: 10.1146/annurev.mi.36.100182.001441. [DOI] [PubMed] [Google Scholar]
- Newman B. M., Cole J. A. The chromosomal location and pleiotropic effects of mutations of the nirA+ gene of Escherichia coli K12: the essential role of nirA+ in nitrite reduction and in other anaerobic redox reactions. J Gen Microbiol. 1978 May;106(1):1–12. doi: 10.1099/00221287-106-1-1. [DOI] [PubMed] [Google Scholar]
- Postle K., Good R. F. A bidirectional rho-independent transcription terminator between the E. coli tonB gene and an opposing gene. Cell. 1985 Jun;41(2):577–585. doi: 10.1016/s0092-8674(85)80030-1. [DOI] [PubMed] [Google Scholar]
- Postle K., Good R. F. DNA sequence of the Escherichia coli tonB gene. Proc Natl Acad Sci U S A. 1983 Sep;80(17):5235–5239. doi: 10.1073/pnas.80.17.5235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruss G. J., Drlica K. Topoisomerase I mutants: the gene on pBR322 that encodes resistance to tetracycline affects plasmid DNA supercoiling. Proc Natl Acad Sci U S A. 1986 Dec;83(23):8952–8956. doi: 10.1073/pnas.83.23.8952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruss G. J., Manes S. H., Drlica K. Escherichia coli DNA topoisomerase I mutants: increased supercoiling is corrected by mutations near gyrase genes. Cell. 1982 Nov;31(1):35–42. doi: 10.1016/0092-8674(82)90402-0. [DOI] [PubMed] [Google Scholar]
- Pugsley A. P., Reeves P. Characterization of group B colicin-resistant mutants of Escherichia coli K-12: colicin resistance and the role of enterochelin. J Bacteriol. 1976 Jul;127(1):218–228. doi: 10.1128/jb.127.1.218-228.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed R. R., Shibuya G. I., Steitz J. A. Nucleotide sequence of gamma delta resolvase gene and demonstration that its gene product acts as a repressor of transcription. Nature. 1982 Nov 25;300(5890):381–383. doi: 10.1038/300381a0. [DOI] [PubMed] [Google Scholar]
- Richardson S. M., Higgins C. F., Lilley D. M. The genetic control of DNA supercoiling in Salmonella typhimurium. EMBO J. 1984 Aug;3(8):1745–1752. doi: 10.1002/j.1460-2075.1984.tb02041.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanzey B. Modulation of gene expression by drugs affecting deoxyribonucleic acid gyrase. J Bacteriol. 1979 Apr;138(1):40–47. doi: 10.1128/jb.138.1.40-47.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauer M., Hantke K., Braun V. Ferric-coprogen receptor FhuE of Escherichia coli: processing and sequence common to all TonB-dependent outer membrane receptor proteins. J Bacteriol. 1987 May;169(5):2044–2049. doi: 10.1128/jb.169.5.2044-2049.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawers R. G., Jamieson D. J., Higgins C. F., Boxer D. H. Characterization and physiological roles of membrane-bound hydrogenase isoenzymes from Salmonella typhimurium. J Bacteriol. 1986 Oct;168(1):398–404. doi: 10.1128/jb.168.1.398-404.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schäffer S., Hantke K., Braun V. Nucleotide sequence of the iron regulatory gene fur. Mol Gen Genet. 1985;200(1):110–113. doi: 10.1007/BF00383321. [DOI] [PubMed] [Google Scholar]
- Shaw D. J., Rice D. W., Guest J. R. Homology between CAP and Fnr, a regulator of anaerobic respiration in Escherichia coli. J Mol Biol. 1983 May 15;166(2):241–247. doi: 10.1016/s0022-2836(83)80011-4. [DOI] [PubMed] [Google Scholar]
- Sinden R. R., Pettijohn D. E. Chromosomes in living Escherichia coli cells are segregated into domains of supercoiling. Proc Natl Acad Sci U S A. 1981 Jan;78(1):224–228. doi: 10.1073/pnas.78.1.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith M. W., Neidhardt F. C. Proteins induced by aerobiosis in Escherichia coli. J Bacteriol. 1983 Apr;154(1):344–350. doi: 10.1128/jb.154.1.344-350.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith M. W., Neidhardt F. C. Proteins induced by anaerobiosis in Escherichia coli. J Bacteriol. 1983 Apr;154(1):336–343. doi: 10.1128/jb.154.1.336-343.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiro S., Guest J. R. Activation of the lac operon of Escherichia coli by a mutant FNR protein. Mol Microbiol. 1987 Jul;1(1):53–58. doi: 10.1111/j.1365-2958.1987.tb00526.x. [DOI] [PubMed] [Google Scholar]
- Stokes H. W., Hall B. G. Topological repression of gene activity by a transposable element. Proc Natl Acad Sci U S A. 1984 Oct;81(19):6115–6119. doi: 10.1073/pnas.81.19.6115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tse-Dinh Y. C. Regulation of the Escherichia coli DNA topoisomerase I gene by DNA supercoiling. Nucleic Acids Res. 1985 Jul 11;13(13):4751–4763. doi: 10.1093/nar/13.13.4751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J. C. DNA topoisomerases. Annu Rev Biochem. 1985;54:665–697. doi: 10.1146/annurev.bi.54.070185.003313. [DOI] [PubMed] [Google Scholar]
- Worcel A., Burgi E. On the structure of the folded chromosome of Escherichia coli. J Mol Biol. 1972 Nov 14;71(2):127–147. doi: 10.1016/0022-2836(72)90342-7. [DOI] [PubMed] [Google Scholar]
- Yamamoto N., Droffner M. L. Mechanisms determining aerobic or anaerobic growth in the facultative anaerobe Salmonella typhimurium. Proc Natl Acad Sci U S A. 1985 Apr;82(7):2077–2081. doi: 10.1073/pnas.82.7.2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yerkes J. H., Casson L. P., Honkanen A. K., Walker G. C. Anaerobiosis induces expression of ant, a new Escherichia coli locus with a role in anaerobic electron transport. J Bacteriol. 1984 Apr;158(1):180–186. doi: 10.1128/jb.158.1.180-186.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]






