Abstract
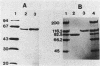
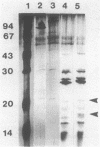
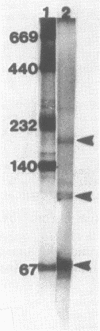
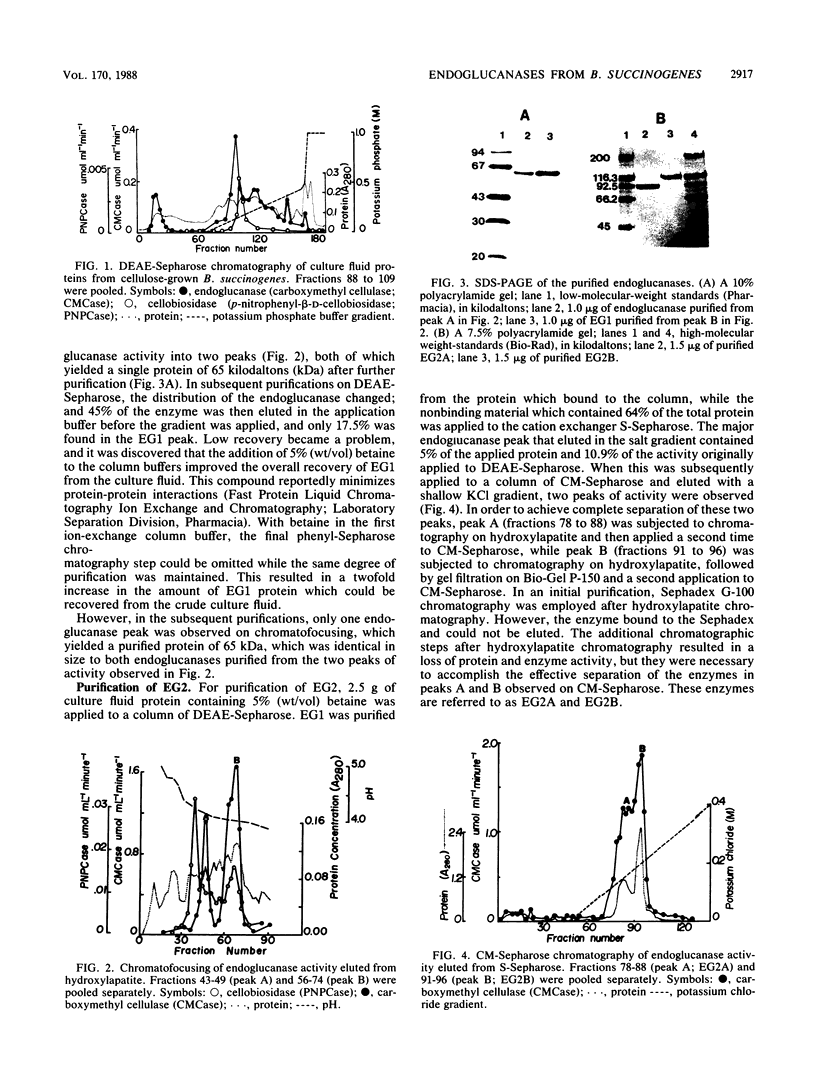
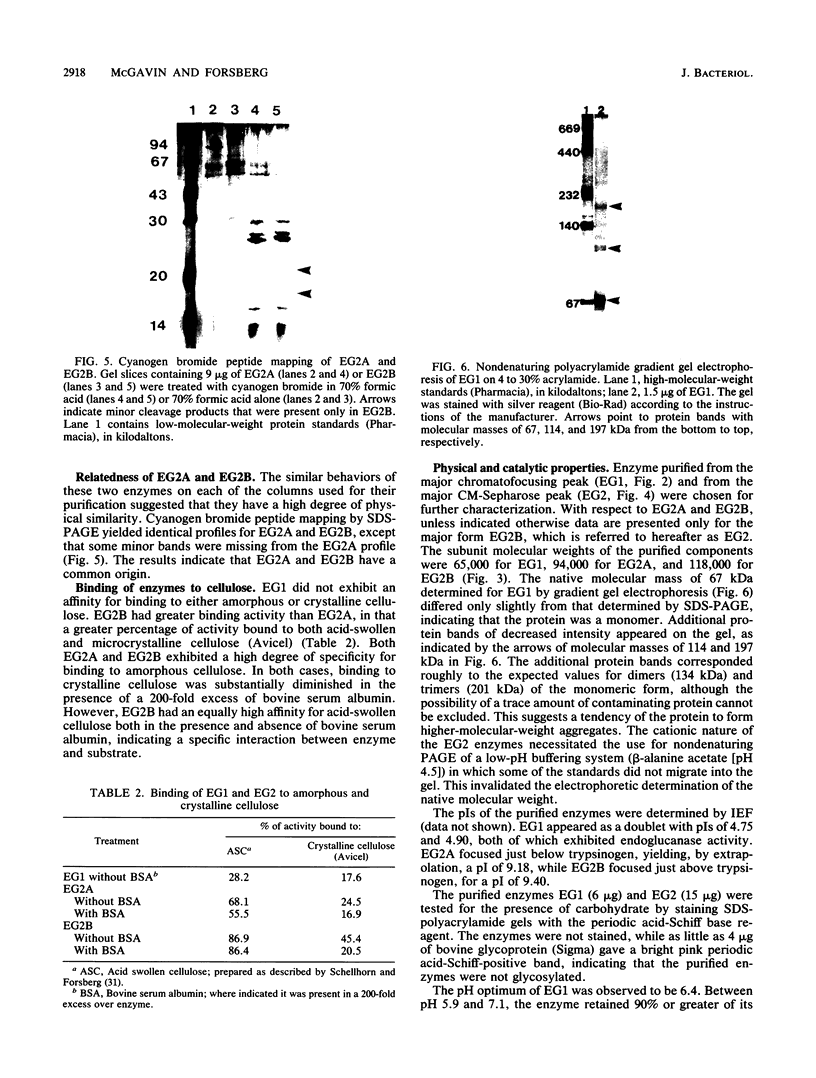
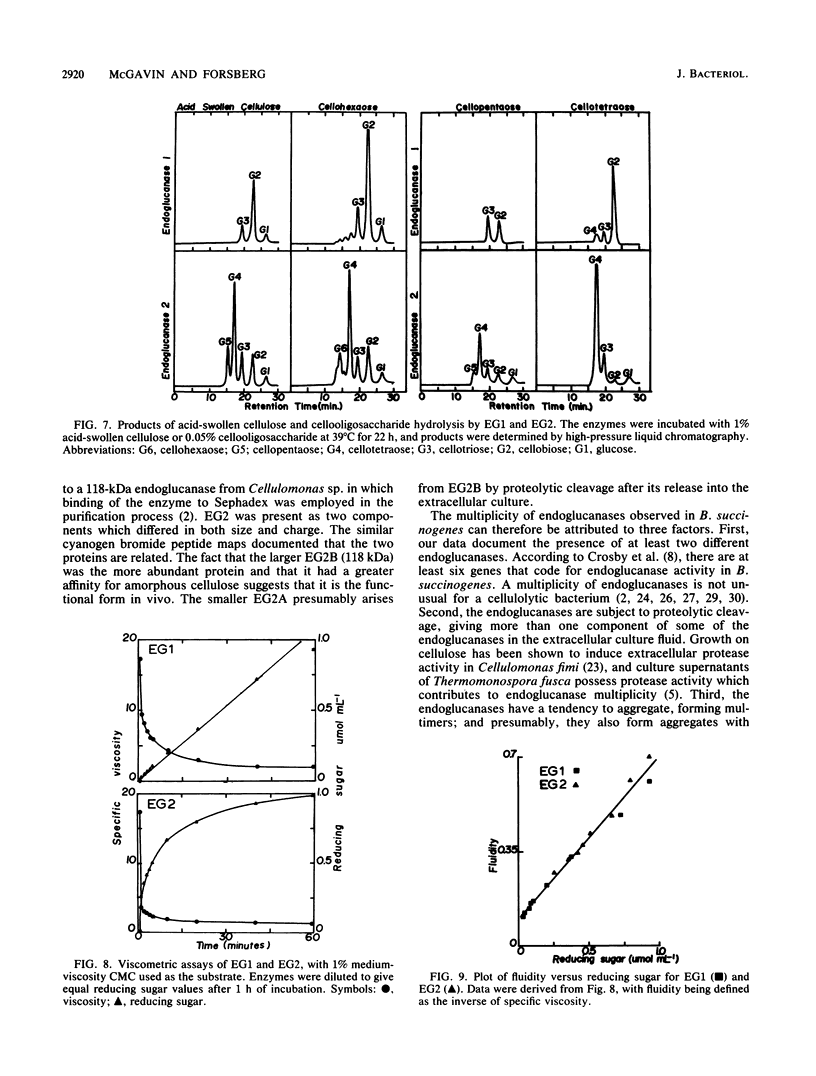
Two endoglucanases designated EG1 and EG2 were purified by column chromatography from the nonsedimentable extracellular culture fluid of Bacteroides succinogenes S85. They accounted for approximately 32 and 11%, respectively, of the total endoglucanase present in the nonsedimentable fraction. The most active enzyme (EG1) had a molecular weight of 65,000, pI of 4.8, and temperature and pH optima of 39 degrees C and 6.4, respectively. The Km for carboxymethyl cellulose was 3.6 mg/ml, and the Vmax was 84 U/mg. The major products of cellulose hydrolysis catalyzed by EG1 were cellotriose and cellobiose. EG2 was present as two components with molecular weights of 118,000 and 94,000. The two components had nearly identical cyanogen bromide peptide maps, thereby indicating that the 94,000-dalton component was a proteolytic degradation product of the 118,000-dalton enzyme. The larger component, which was more abundant in the culture fluid than the smaller form was, had a Km of 12.2 mg/ml and a Vmax of 10.4 U/mg. It was a basic protein with a pI of 9.4, a temperature optimum of 39 degrees C, and a pH optimum of 5.8. The major product of cellulose hydrolysis was cellotetraose. EG2 exhibited specific binding to acid-swollen cellulose, whereas EG1 did not, and neither of them had affinity for crystalline cellulose. Based on the substrate specificities and the affinities of the two enzymes for cellulose, we postulated that EG2 is involved in the early stages of cellulose hydrolysis and that EG1 is active primarily on the products arising from EG2.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Béguin P., Eisen H. Purification and partial characterization of three extracellular cellulases from Cellulomonas sp. Eur J Biochem. 1978 Jul 3;87(3):525–531. doi: 10.1111/j.1432-1033.1978.tb12403.x. [DOI] [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Forsberg C. W., Beveridge T. J., Hellstrom A. Cellulase and Xylanase Release from Bacteroides succinogenes and Its Importance in the Rumen Environment. Appl Environ Microbiol. 1981 Nov;42(5):886–896. doi: 10.1128/aem.42.5.886-896.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaudet G. Les cellulases extracellulaires de Bacteroides succinogenes. Ann Microbiol (Paris) 1983 Jan-Feb;134A(1):111–114. [PubMed] [Google Scholar]
- Gilkes N. R., Langsford M. L., Kilburn D. G., Miller R. C., Jr, Warren R. A. Mode of action and substrate specificities of cellulases from cloned bacterial genes. J Biol Chem. 1984 Aug 25;259(16):10455–10459. [PubMed] [Google Scholar]
- Groleau D., Forsberg C. W. Cellulolytic activity of the rumen bacterium Bacteroides succinogenes. Can J Microbiol. 1981 May;27(5):517–530. doi: 10.1139/m81-077. [DOI] [PubMed] [Google Scholar]
- Groleau D., Forsberg C. W. Partial characterization of the extracellular carboxymethylcellulase activity produced by the rumen bacterium Bacteroides succinogenes. Can J Microbiol. 1983 May;29(5):504–517. doi: 10.1139/m83-080. [DOI] [PubMed] [Google Scholar]
- Huang L., Forsberg C. W. Isolation of a Cellodextrinase from Bacteroides succinogenes. Appl Environ Microbiol. 1987 May;53(5):1034–1041. doi: 10.1128/aem.53.5.1034-1041.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang L., Forsberg C. W., Thomas D. Y. Purification and characterization of a chloride-stimulated cellobiosidase from Bacteroides succinogenes S85. J Bacteriol. 1988 Jul;170(7):2923–2932. doi: 10.1128/jb.170.7.2923-2932.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lin E., Wilson D. B. Regulation of beta-1,4-Endoglucanase Synthesis in Thermomonospora fusca. Appl Environ Microbiol. 1987 Jun;53(6):1352–1357. doi: 10.1128/aem.53.6.1352-1357.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng T. K., Zeikus J. G. Purification and characterization of an endoglucanase (1,4-beta-D-glucan glucanohydrolase) from Clostridium thermocellum. Biochem J. 1981 Nov 1;199(2):341–350. doi: 10.1042/bj1990341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petre J., Longin R., Millet J. Purification and properties of an endo-beta-1,4-glucanase from Clostridium thermocellum. Biochimie. 1981 Jul;63(7):629–639. doi: 10.1016/s0300-9084(81)80061-2. [DOI] [PubMed] [Google Scholar]
- Pétré D., Millet J., Longin R., Béguin P., Girard H., Aubert J. P. Purification and properties of the endoglucanase C of Clostridium thermocellum produced in Escherichia coli. Biochimie. 1986 May;68(5):687–695. doi: 10.1016/s0300-9084(86)80162-6. [DOI] [PubMed] [Google Scholar]
- Romaniec M. P., Clarke N. G., Hazlewood G. P. Molecular cloning of Clostridium thermocellum DNA and the expression of further novel endo-beta-1,4-glucanase genes in Escherichia coli. J Gen Microbiol. 1987 May;133(5):1297–1307. doi: 10.1099/00221287-133-5-1297. [DOI] [PubMed] [Google Scholar]
- Shoemaker S. P., Brown R. D., Jr Enzymic activities of endo-1,4-beta-D-glucanases purified from Trichoderma viride. Biochim Biophys Acta. 1978 Mar 14;523(1):133–146. doi: 10.1016/0005-2744(78)90016-5. [DOI] [PubMed] [Google Scholar]
- Stack R. J., Hungate R. E. Effect of 3-Phenylpropanoic Acid on Capsule and Cellulases of Ruminococcus albus 8. Appl Environ Microbiol. 1984 Jul;48(1):218–223. doi: 10.1128/aem.48.1.218-223.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor K. A., Crosby B., McGavin M., Forsberg C. W., Thomas D. Y. Characteristics of the endoglucanase encoded by a cel gene from Bacteroides succinogenes expressed in Escherichia coli. Appl Environ Microbiol. 1987 Jan;53(1):41–46. doi: 10.1128/aem.53.1.41-46.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHITAKER D. R. Hydrolysis of a series of beta-1,4'-oligoglucosides by Myrothecium verrucaria cellulase. Arch Biochem Biophys. 1954 Dec;53(2):439–449. doi: 10.1016/0003-9861(54)90425-7. [DOI] [PubMed] [Google Scholar]
- Wood T. M. Properties of cellulolytic enzyme systems. Biochem Soc Trans. 1985 Apr;13(2):407–410. doi: 10.1042/bst0130407. [DOI] [PubMed] [Google Scholar]