Abstract
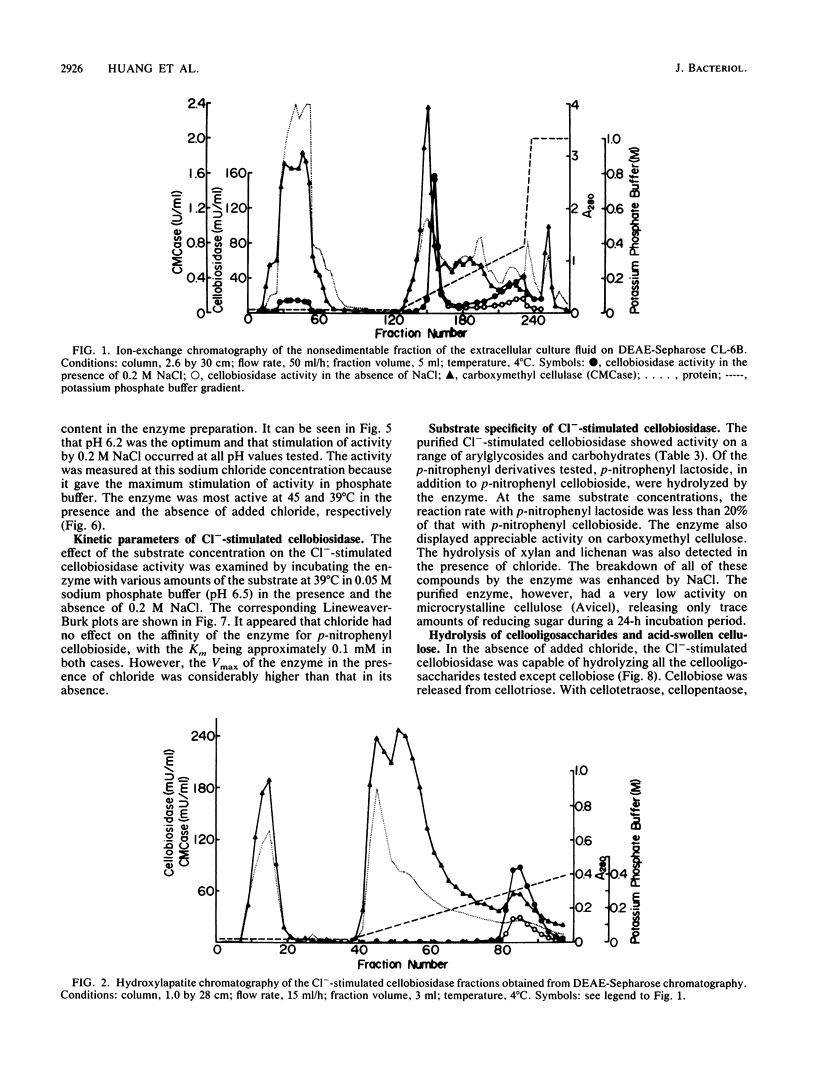
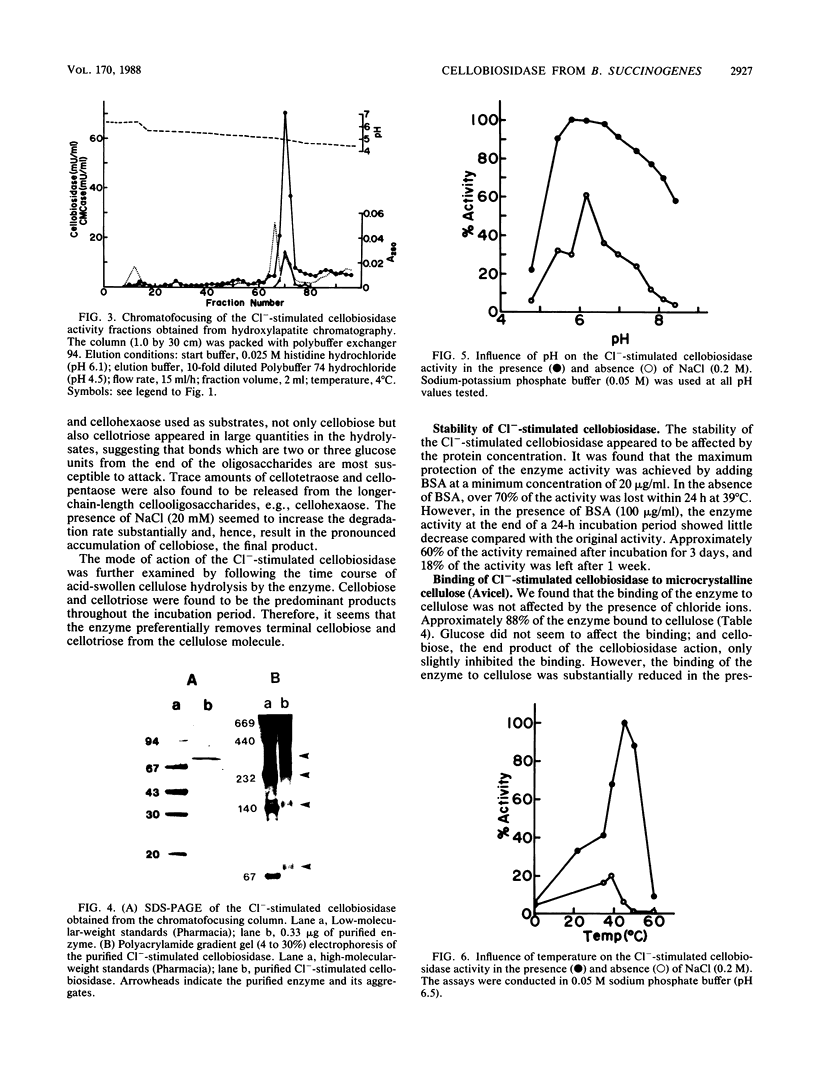
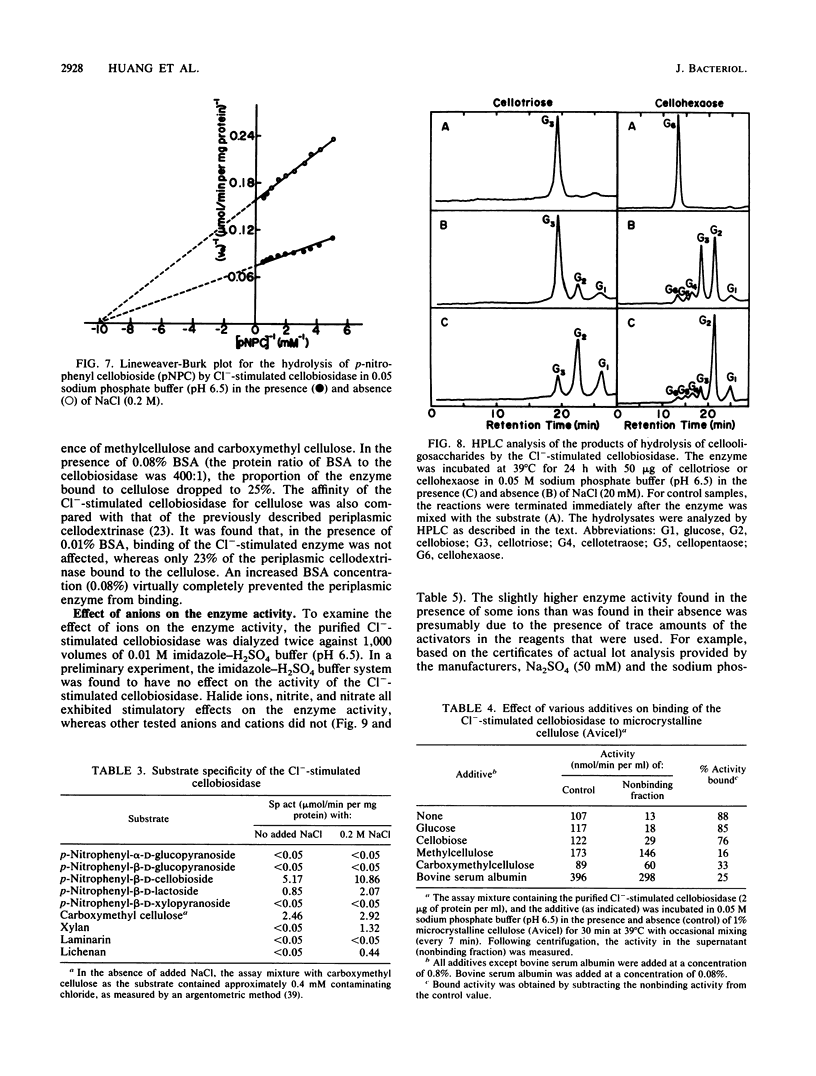
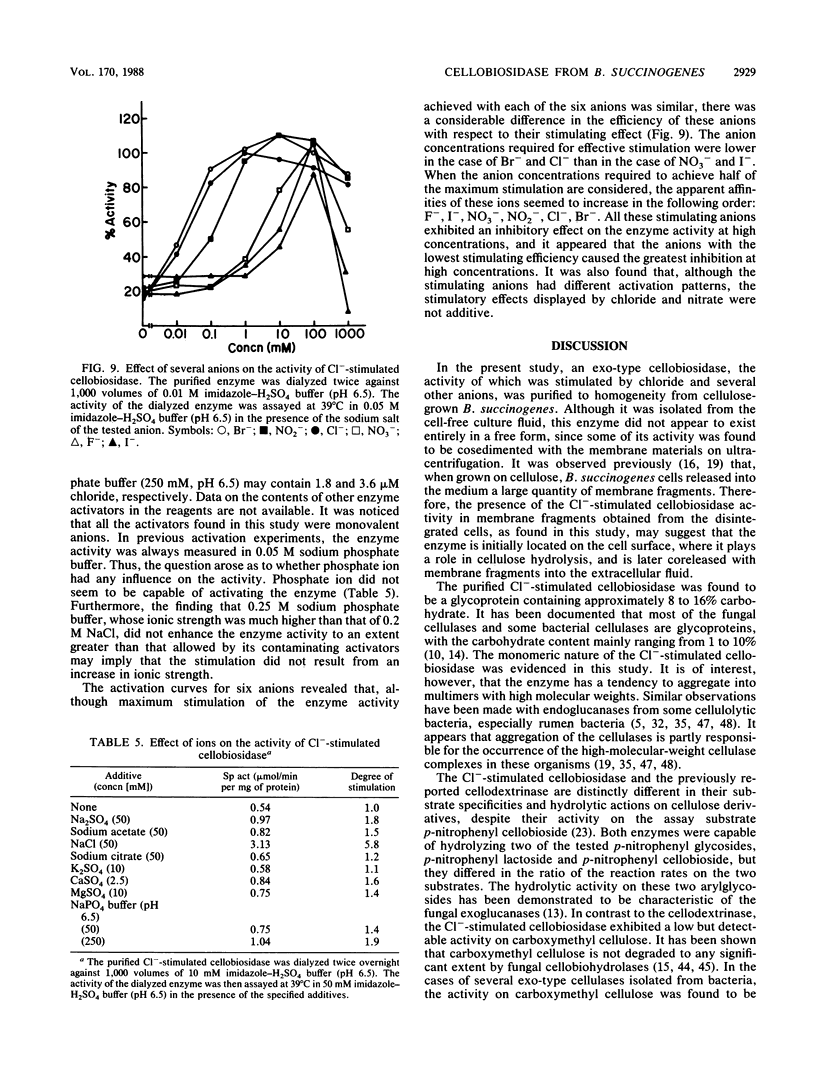
A cellobiosidase with unique characteristics from the extracellular culture fluid of the anaerobic gram-negative cellulolytic rumen bacterium Bacteroides succinogenes grown on microcrystalline cellulose (Avicel) in a continuous culture system was purified to homogeneity by column chromatography. The enzyme was a glycoprotein with a molecular weight of approximately 75,000 and an isoelectric point of 6.7. When assayed at 39 degrees C and pH 6.5, the activity of the enzyme with p-nitrophenyl-beta-D-cellobioside as the substrate was stimulated by chloride, bromide, fluoride, iodide, nitrate, and nitrite, with maximum activation (approximately sevenfold) occurring at concentrations ranging from 1.0 mM (Cl-) to greater than 0.75 M (F-). The presence of chloride (0.2 M) did not affect the Km but doubled the Vmax. In the presence of chloride (0.2 M), the pH optimum of the enzyme was broadened, and the temperature optimum was increased from 39 to 45 degrees C. The enzyme released terminal cellobiose from cellotriose and cellobiose and cellotriose from longer-chain-length cellooligosaccharrides and acid-swollen cellulose, but it had no activity on cellobiose. The enzyme showed affinity for cellulose (Avicel) but did not hydrolyze it. It also had a low activity on carboxymethyl cellulose.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahlers J., Günther T. Kinetics of the ion-sensitive Mg, Ca-adenosinetriphosphatase (ATPase) from Escherichia coli. Arch Biochem Biophys. 1975 Nov;171(1):163–169. doi: 10.1016/0003-9861(75)90019-3. [DOI] [PubMed] [Google Scholar]
- BEZKOROVAINY A., DOHERTY D. G. Comparison of bovine and human orosomucoids. Nature. 1962 Sep 8;195:1003–1003. doi: 10.1038/1951003a0. [DOI] [PubMed] [Google Scholar]
- Bennink M. R., Tyler T. R., Ward G. M., Johnson D. E. Ionic milieu of bovine and ovine rumen as affected by diet. J Dairy Sci. 1978 Mar;61(3):315–323. doi: 10.3168/jds.S0022-0302(78)83600-5. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Bünning P., Riordan J. F. Activation of angiotensin converting enzyme by monovalent anions. Biochemistry. 1983 Jan 4;22(1):110–116. doi: 10.1021/bi00270a016. [DOI] [PubMed] [Google Scholar]
- Cole S. W. Contributions to our knowledge of the action of enzymes: Part I. The influence of electrolytes on the action of amylolytic ferments. J Physiol. 1903 Nov 2;30(2):202–220. doi: 10.1113/jphysiol.1903.sp000990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craine J. E., Daniels G. H., Kaufman S. Dopamine-beta-hydroxylase. The subunit structure and anion activation of the bovine adrenal enzyme. J Biol Chem. 1973 Nov 25;248(22):7838–7844. [PubMed] [Google Scholar]
- Deshpande M. V., Eriksson K. E., Pettersson L. G. An assay for selective determination of exo-1,4,-beta-glucanases in a mixture of cellulolytic enzymes. Anal Biochem. 1984 May 1;138(2):481–487. doi: 10.1016/0003-2697(84)90843-1. [DOI] [PubMed] [Google Scholar]
- Eriksson K. E., Pettersson B. Extracellular enzyme system utilized by the fungus Sporotrichum pulverulentum (Chrysosporium lignorum) for the breakdown of cellulose. 3. Purification and physico-chemical characterization of an exo-1,4-beta-glucanase. Eur J Biochem. 1975 Feb 3;51(1):213–218. doi: 10.1111/j.1432-1033.1975.tb03921.x. [DOI] [PubMed] [Google Scholar]
- Forsberg C. W., Beveridge T. J., Hellstrom A. Cellulase and Xylanase Release from Bacteroides succinogenes and Its Importance in the Rumen Environment. Appl Environ Microbiol. 1981 Nov;42(5):886–896. doi: 10.1128/aem.42.5.886-896.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorter J., Gruber M. Cathepsin C: an allosteric enzyme. Biochim Biophys Acta. 1970 Mar 18;198(3):546–555. doi: 10.1016/0005-2744(70)90132-4. [DOI] [PubMed] [Google Scholar]
- Groleau D., Forsberg C. W. Cellulolytic activity of the rumen bacterium Bacteroides succinogenes. Can J Microbiol. 1981 May;27(5):517–530. doi: 10.1139/m81-077. [DOI] [PubMed] [Google Scholar]
- Groleau D., Forsberg C. W. Partial characterization of the extracellular carboxymethylcellulase activity produced by the rumen bacterium Bacteroides succinogenes. Can J Microbiol. 1983 May;29(5):504–517. doi: 10.1139/m83-080. [DOI] [PubMed] [Google Scholar]
- Gum E. K., Jr, Brown R. D., Jr Comparison of four purified extracellular 1,4-beta-D-glucan cellobiohydrolase enzymes from Trichoderma viride. Biochim Biophys Acta. 1977 May 27;492(1):225–231. doi: 10.1016/0005-2795(77)90229-x. [DOI] [PubMed] [Google Scholar]
- Huang L., Forsberg C. W. Isolation of a Cellodextrinase from Bacteroides succinogenes. Appl Environ Microbiol. 1987 May;53(5):1034–1041. doi: 10.1128/aem.53.5.1034-1041.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalish M. I., Pineyro M. A., Cooper B., Gregerman R. I. Adenylyl cyclase activation by halide anions other than fluoride. Biochem Biophys Res Commun. 1974 Nov 27;61(2):781–787. doi: 10.1016/0006-291x(74)91025-0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lamed R., Naimark J., Morgenstern E., Bayer E. A. Specialized cell surface structures in cellulolytic bacteria. J Bacteriol. 1987 Aug;169(8):3792–3800. doi: 10.1128/jb.169.8.3792-3800.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latham M. J., Brooker B. E., Pettipher G. L., Harris P. J. Adhesion of Bacteroides succinogenes in pure culture and in the presence of Ruminococcus flavefaciens to cell walls in leaves of perennial ryegrass (Lolium perenne). Appl Environ Microbiol. 1978 Jun;35(6):1166–1173. doi: 10.1128/aem.35.6.1166-1173.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitzki A., Steer M. L. The allosteric activation of mammalian alpha-amylase by chloride. Eur J Biochem. 1974 Jan 3;41(1):171–180. doi: 10.1111/j.1432-1033.1974.tb03257.x. [DOI] [PubMed] [Google Scholar]
- MacKenzie C. R., Bilous D., Johnson K. G. Purification and characterization of an exoglucanase from Streptomyces flavogriseus. Can J Microbiol. 1984 Sep;30(9):1171–1178. doi: 10.1139/m84-183. [DOI] [PubMed] [Google Scholar]
- McGavin M., Forsberg C. W. Isolation and characterization of endoglucanases 1 and 2 from Bacteroides succinogenes S85. J Bacteriol. 1988 Jul;170(7):2914–2922. doi: 10.1128/jb.170.7.2914-2922.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHale A., Coughlan M. P. Synergistic hydrolysis of cellulose by components of the extracellular cellulase system of Talaromyces emersonii. FEBS Lett. 1980 Aug 11;117(1):319–322. doi: 10.1016/0014-5793(80)80971-9. [DOI] [PubMed] [Google Scholar]
- Ohmiya K., Shimizu M., Taya M., Shimizu S. Purification and properties of cellobiosidase from Ruminococcus albus. J Bacteriol. 1982 Apr;150(1):407–409. doi: 10.1128/jb.150.1.407-409.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SKEGGS L. T., Jr, MARSH W. H., KAHN J. R., SHUMWAY N. P. The existence of two forms of hypertensin. J Exp Med. 1954 Mar;99(3):275–282. doi: 10.1084/jem.99.3.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor K. A., Crosby B., McGavin M., Forsberg C. W., Thomas D. Y. Characteristics of the endoglucanase encoded by a cel gene from Bacteroides succinogenes expressed in Escherichia coli. Appl Environ Microbiol. 1987 Jan;53(1):41–46. doi: 10.1128/aem.53.1.41-46.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEBB E. C., MORROW P. F. The activation of an arysulphatase from ox liver by chloride and other anions. Biochem J. 1959 Sep;73:7–15. doi: 10.1042/bj0730007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood T. M., McCrae S. I. The purification and properties of the C 1 component of Trichoderma koningii cellulase. Biochem J. 1972 Aug;128(5):1183–1192. doi: 10.1042/bj1281183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood T. M. Properties of cellulolytic enzyme systems. Biochem Soc Trans. 1985 Apr;13(2):407–410. doi: 10.1042/bst0130407. [DOI] [PubMed] [Google Scholar]
- Wood T. M., Wilson C. A., Stewart C. S. Preparation of the cellulase from the cellulolytic anaerobic rumen bacterium Ruminococcus albus and its release from the bacterial cell wall. Biochem J. 1982 Jul 1;205(1):129–137. doi: 10.1042/bj2050129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu I., Hungate R. E. The extracellular cellulases of Ruminococcus albus. Ann Rech Vet. 1979;10(2-3):251–254. [PubMed] [Google Scholar]



