Abstract
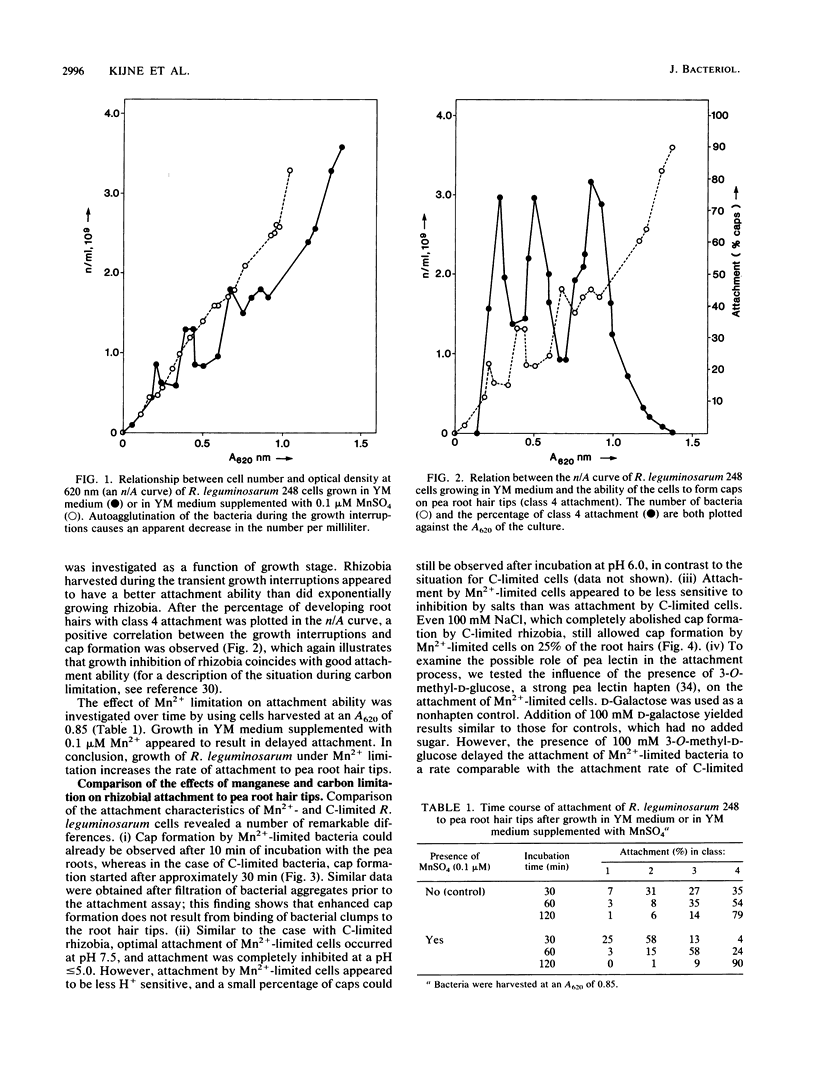
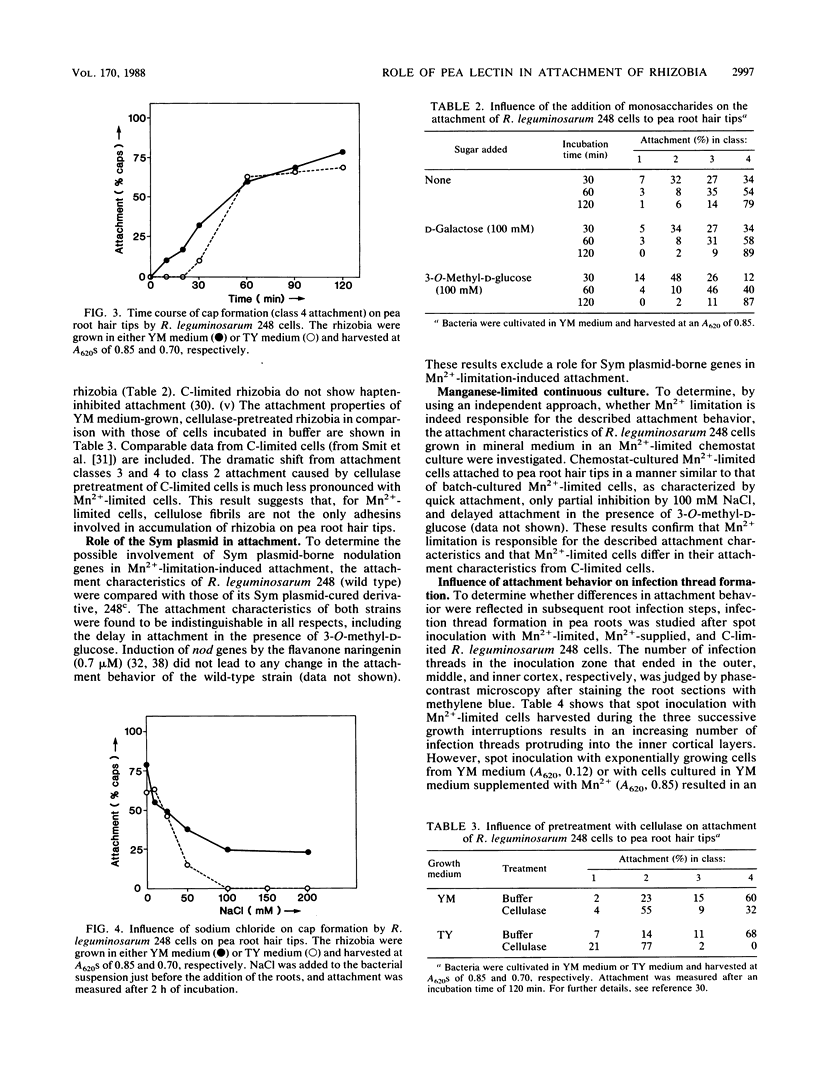
The ability of Rhizobium leguminosarum 248 to attach to developing Pisum sativum root hairs was investigated during various phases of bacterial growth in yeast extract-mannitol medium. Direct cell counting revealed that growth of the rhizobia transiently stopped three successive times during batch culture in yeast extract-mannitol medium. These interruptions of growth, as well as the simultaneous autoagglutination of the bacteria, appeared to be caused by manganese limitation. Rhizobia harvested during the transient phases of growth inhibition appeared to have a better attachment ability than did exponentially growing rhizobia. The attachment characteristics of these manganese-limited rhizobia were compared with those of carbon-limited rhizobia (G. Smit, J. W. Kijne, and B. J. J. Lugtenberg, J. Bacteriol. 168:821-827, 1986, and J. Bacteriol. 169:4294-4301, 1987). In contrast to the attachment of carbon-limited cells, accumulation of manganese-limited rhizobia (cap formation) was already in full progress after 10 min of incubation; significantly delayed by 3-O-methyl-D-glucose, a pea lectin haptenic monosaccharide; partially resistant to sodium chloride; and partially resistant to pretreatment of the bacteria with cellulase. Binding of single bacteria to the root hair tips was not inhibited by 3-O-methyl-D-glucose. Whereas attachment of single R. leguminosarum cells to the surface of pea root hair tips seemed to be similar for both carbon- and manganese-limited cells, the subsequent accumulation of manganese-limited rhizobia at the root hair tips is apparently accelerated by pea lectin molecules. Moreover, spot inoculation tests with rhizobia grown under various culture conditions indicated that differences in attachment between manganese- and carbon-limited R. leguminosarum cells are correlated with a significant difference in infectivity in that manganese-limited rhizobia, in contrast to carbon-limited rhizobia, are infective. This growth-medium-dependent behavior offers and explanation for the seemingly conflicting data on the involvement of host plant lectins in attachment of rhizobia to root hairs of leguminous plants. Sym plasmid-borne genes do not play a role in manganese-limitation-induced attachment of R. leguminosarum.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abe M., Sherwood J. E., Hollingsworth R. I., Dazzo F. B. Stimulation of clover root hair infection by lectin-binding oligosaccharides from the capsular and extracellular polysaccharides of Rhizobium trifolii. J Bacteriol. 1984 Nov;160(2):517–520. doi: 10.1128/jb.160.2.517-520.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badenoch-Jones J., Flanders D. J., Rolfe B. G. Association of Rhizobium Strains with Roots of Trifolium repens. Appl Environ Microbiol. 1985 Jun;49(6):1511–1520. doi: 10.1128/aem.49.6.1511-1520.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beringer J. E. R factor transfer in Rhizobium leguminosarum. J Gen Microbiol. 1974 Sep;84(1):188–198. doi: 10.1099/00221287-84-1-188. [DOI] [PubMed] [Google Scholar]
- Caetano Anollés G., Favelukes G. Host-Symbiont Specificity Expressed during Early Adsorption of Rhizobium meliloti to the Root Surface of Alfalfa. Appl Environ Microbiol. 1986 Aug;52(2):377–382. doi: 10.1128/aem.52.2.377-382.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caetano Anollés G., Favelukes G. Quantitation of adsorption of rhizobia in low numbers to small legume roots. Appl Environ Microbiol. 1986 Aug;52(2):371–376. doi: 10.1128/aem.52.2.371-376.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dazzo F. B. Bacterial attachment as related to cellular recognition in the Rhizobium-legume symbiosis. J Supramol Struct Cell Biochem. 1981;16(1):29–41. doi: 10.1002/jsscb.1981.380160104. [DOI] [PubMed] [Google Scholar]
- Dazzo F. B., Brill W. J. Bacterial polysaccharide which binds Rhizobium trifolii to clover root hairs. J Bacteriol. 1979 Mar;137(3):1362–1373. doi: 10.1128/jb.137.3.1362-1373.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dazzo F. B., Hubbell D. H. Cross-reactive antigens and lectin as determinants of symbiotic specificity in the Rhizobium-clover association. Appl Microbiol. 1975 Dec;30(6):1017–1033. doi: 10.1128/am.30.6.1017-1033.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dazzo F. B., Napoli C. A., Hubbell D. H. Adsorption of bacteria to roots as related to host specificity in the Rhizobium-clover symbiosis. Appl Environ Microbiol. 1976 Jul;32(1):166–171. doi: 10.1128/aem.32.1.166-171.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halverson L. J., Stacey G. Signal exchange in plant-microbe interactions. Microbiol Rev. 1986 Jun;50(2):193–225. doi: 10.1128/mr.50.2.193-225.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D., Hubbell D. H. Infection thread formation as a basis of nodulation specificity in Rhizobium--strawberry clover associations. Can J Microbiol. 1969 Oct;15(10):1133–1136. doi: 10.1139/m69-206. [DOI] [PubMed] [Google Scholar]
- Mort A. J., Bauer W. D. Application of two new methods for cleavage of polysaccharides into specific oligosaccharide fragments. Structure of the capsular and extracellular polysaccharides of Rhizobium japonicum that bind soybean lectin. J Biol Chem. 1982 Feb 25;257(4):1870–1875. [PubMed] [Google Scholar]
- Mort A. J., Bauer W. D. Composition of the Capsular and Extracellular Polysaccharides of Rhizobium japonicum: CHANGES WITH CULTURE AGE AND CORRELATIONS WITH BINDING OF SOYBEAN SEED LECTIN TO THE BACTERIA . Plant Physiol. 1980 Jul;66(1):158–163. doi: 10.1104/pp.66.1.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters N. K., Frost J. W., Long S. R. A plant flavone, luteolin, induces expression of Rhizobium meliloti nodulation genes. Science. 1986 Aug 29;233(4767):977–980. doi: 10.1126/science.3738520. [DOI] [PubMed] [Google Scholar]
- Planqué K., Kijne J. W. Binding of pea lectins to a glycan type polysaccharide in the cell walls of Rhizobium leguminosarum. FEBS Lett. 1977 Jan 15;73(1):64–66. doi: 10.1016/0014-5793(77)80016-1. [DOI] [PubMed] [Google Scholar]
- Smit G., Kijne J. W., Lugtenberg B. J. Correlation between extracellular fibrils and attachment of Rhizobium leguminosarum to pea root hair tips. J Bacteriol. 1986 Nov;168(2):821–827. doi: 10.1128/jb.168.2.821-827.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smit G., Kijne J. W., Lugtenberg B. J. Involvement of both cellulose fibrils and a Ca2+-dependent adhesin in the attachment of Rhizobium leguminosarum to pea root hair tips. J Bacteriol. 1987 Sep;169(9):4294–4301. doi: 10.1128/jb.169.9.4294-4301.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolpert J. S., Albersheim P. Host-symbiont interactions. I. The lectins of legumes interact with the o-antigen-containing lipopolysaccharides of their symbiont Rhizobia. Biochem Biophys Res Commun. 1976 Jun 7;70(3):729–737. doi: 10.1016/0006-291x(76)90653-7. [DOI] [PubMed] [Google Scholar]
- Zaat S. A., Wijffelman C. A., Spaink H. P., van Brussel A. A., Okker R. J., Lugtenberg B. J. Induction of the nodA promoter of Rhizobium leguminosarum Sym plasmid pRL1JI by plant flavanones and flavones. J Bacteriol. 1987 Jan;169(1):198–204. doi: 10.1128/jb.169.1.198-204.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zurkowski W. Specific adsorption of bacteria to clover root hairs, related to the presence of the plasmid pWZ2 in cells of Rhizobium trifolii. Microbios. 1980;27(107):27–32. [PubMed] [Google Scholar]
- van der Schaal I. A., Logman T. J., Díaz C. L., Kijne J. W. An enzyme-linked lectin binding assay for quantitative determination of lectin receptors. Anal Biochem. 1984 Jul;140(1):48–55. doi: 10.1016/0003-2697(84)90131-3. [DOI] [PubMed] [Google Scholar]


