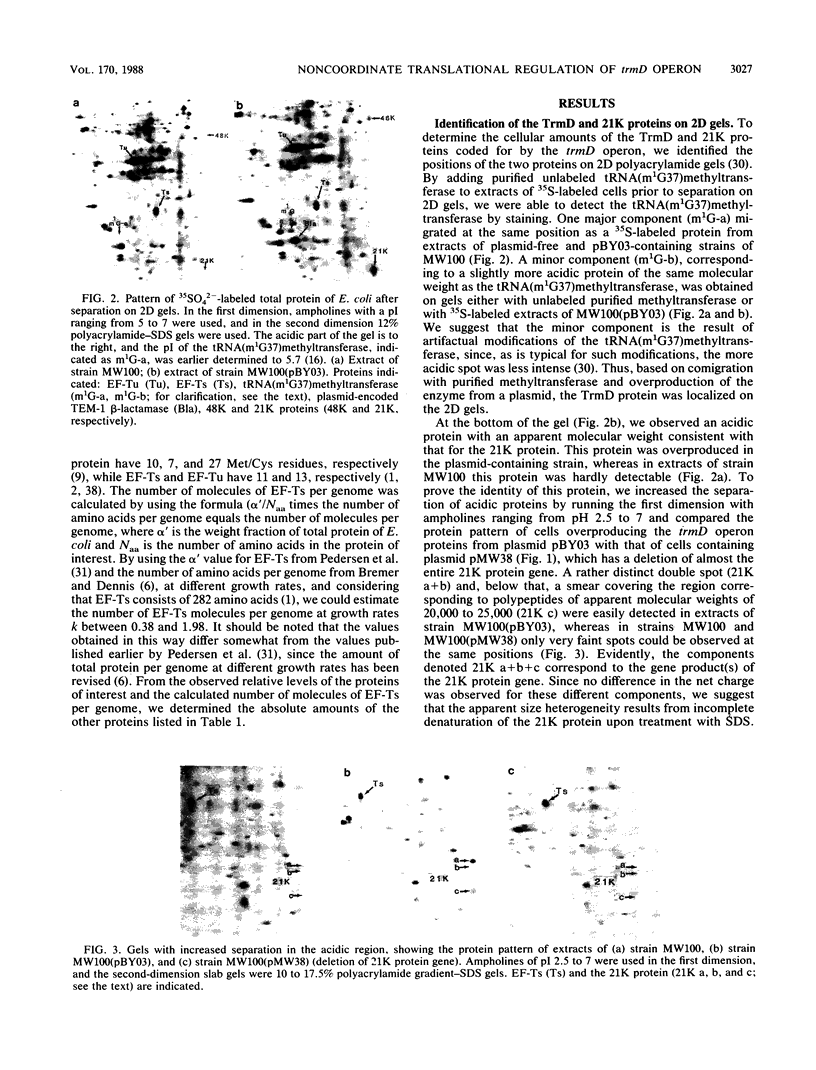
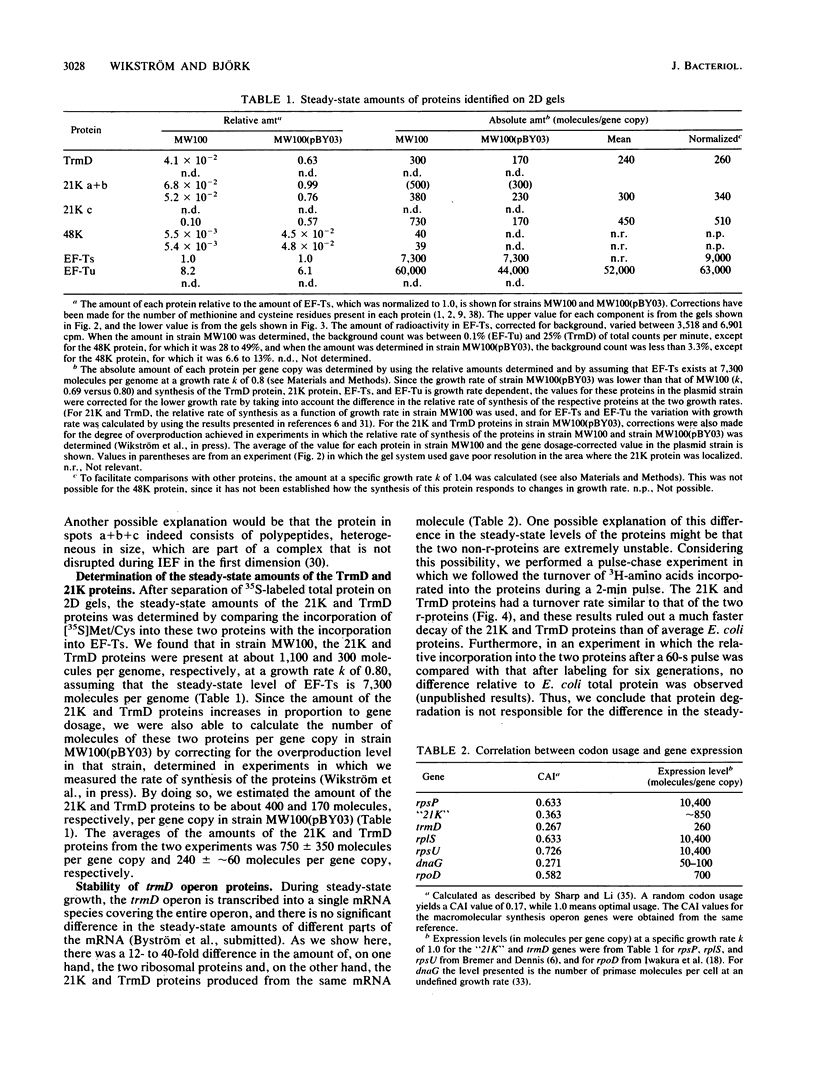
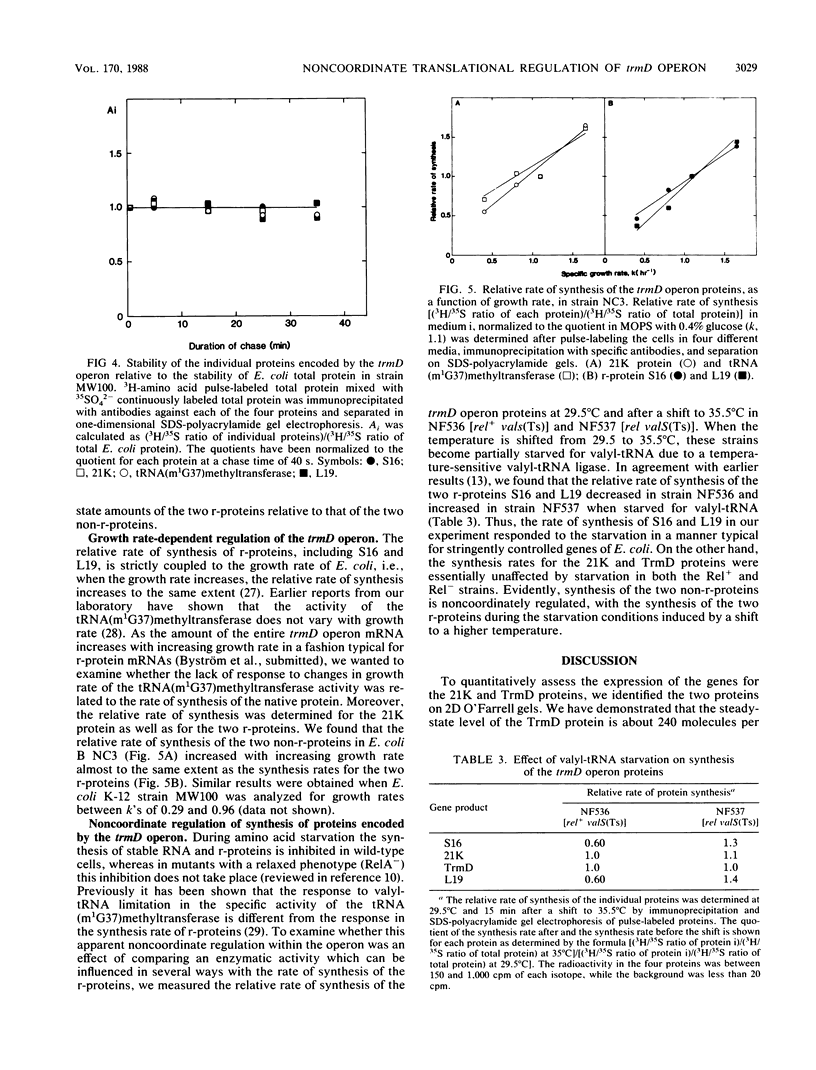
Abstract
The trmD operon of Escherichia coli contains the genes for the ribosomal protein S16, a 21-kilodalton polypeptide of unknown function, the tRNA(1-methylguanosine)methyltransferase, and the ribosomal protein L19, in that order. As reported elsewhere, the operon is transcribed as a single polycistronic mRNA species, and there is no significant difference in the steady-state amounts of different parts of the mRNA (A.S. Byström, A. von Gabain, and G.R. Björk, submitted for publication). Furthermore, accumulation of all parts of the transcript is altered in a stringently controlled manner upon starvation for valyl-tRNA. Here we show that the rate of synthesis of the trmD operon proteins increased with increasing growth rate and that the amount in steady state, at a specific growth rate (k = 1.0), of the tRNA(1-methylguanosine)methyltransferase was 260 molecules per gene copy, which is about 40 times lower than the amount of the two ribosomal proteins, whereas the 21-kilodalton protein was synthesized to the amount of about 850 molecules per gene copy. The lower steady-state amount of the two nonribosomal proteins was not due to a higher turnover rate. Synthesis of the 21-kilodalton and TrmD proteins responded differently from that of the two ribosomal proteins during conditions which provoked amino acid starvation, although accumulation of the entire mRNA molecule responds similarly to the rate of synthesis of the two ribosomal proteins. We conclude that the observed differential and noncoordinate expression is achieved by regulation at the level of mRNA translation.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- An G., Bendiak D. S., Mamelak L. A., Friesen J. D. Organization and nucleotide sequence of a new ribosomal operon in Escherichia coli containing the genes for ribosomal protein S2 and elongation factor Ts. Nucleic Acids Res. 1981 Aug 25;9(16):4163–4172. doi: 10.1093/nar/9.16.4163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An G., Friesen J. D. The nucleotide sequence of tufB and four nearby tRNA structural genes of Escherichia coli. Gene. 1980 Dec;12(1-2):33–39. doi: 10.1016/0378-1119(80)90013-x. [DOI] [PubMed] [Google Scholar]
- Björk G. R. E. coli ribosomal protein operons: the case of the misplaced genes. Cell. 1985 Aug;42(1):7–8. doi: 10.1016/s0092-8674(85)80093-3. [DOI] [PubMed] [Google Scholar]
- Björk G. R., Ericson J. U., Gustafsson C. E., Hagervall T. G., Jönsson Y. H., Wikström P. M. Transfer RNA modification. Annu Rev Biochem. 1987;56:263–287. doi: 10.1146/annurev.bi.56.070187.001403. [DOI] [PubMed] [Google Scholar]
- Byström A. S., Björk G. R. Chromosomal location and cloning of the gene (trmD) responsible for the synthesis of tRNA (m1G) methyltransferase in Escherichia coli K-12. Mol Gen Genet. 1982;188(3):440–446. doi: 10.1007/BF00330046. [DOI] [PubMed] [Google Scholar]
- Byström A. S., Björk G. R. The structural gene (trmD) for the tRNA(m1G)methyltransferase is part of a four polypeptide operon in Escherichia coli K-12. Mol Gen Genet. 1982;188(3):447–454. doi: 10.1007/BF00330047. [DOI] [PubMed] [Google Scholar]
- Byström A. S., Hjalmarsson K. J., Wikström P. M., Björk G. R. The nucleotide sequence of an Escherichia coli operon containing genes for the tRNA(m1G)methyltransferase, the ribosomal proteins S16 and L19 and a 21-K polypeptide. EMBO J. 1983;2(6):899–905. doi: 10.1002/j.1460-2075.1983.tb01519.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole J. R., Nomura M. Translational regulation is responsible for growth-rate-dependent and stringent control of the synthesis of ribosomal proteins L11 and L1 in Escherichia coli. Proc Natl Acad Sci U S A. 1986 Jun;83(12):4129–4133. doi: 10.1073/pnas.83.12.4129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis P. P., Nomura M. Stringent control of ribosomal protein gene expression in Escherichia coli. Proc Natl Acad Sci U S A. 1974 Oct;71(10):3819–3823. doi: 10.1073/pnas.71.10.3819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis P. P., Nomura M. Stringent control of the transcriptional activities of ribosomal protein genes in E. coli. Nature. 1975 Jun 5;255(5508):460–465. doi: 10.1038/255460a0. [DOI] [PubMed] [Google Scholar]
- Freedman L. P., Zengel J. M., Lindahl L. Genetic dissection of stringent control and nutritional shift-up response of the Escherichia coli S10 ribosomal protein operon. J Mol Biol. 1985 Oct 20;185(4):701–712. doi: 10.1016/0022-2836(85)90055-5. [DOI] [PubMed] [Google Scholar]
- Hjalmarsson K. J., Byström A. S., Björk G. R. Purification and characterization of transfer RNA (guanine-1)methyltransferase from Escherichia coli. J Biol Chem. 1983 Jan 25;258(2):1343–1351. [PubMed] [Google Scholar]
- Holm L. Codon usage and gene expression. Nucleic Acids Res. 1986 Apr 11;14(7):3075–3087. doi: 10.1093/nar/14.7.3075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwakura Y., Ito K., Ishihama A. Biosynthesis of RNA polymerase in Escherichia coli. I. Control of RNA polymerase content at various growth rates. Mol Gen Genet. 1974;133(1):1–23. doi: 10.1007/BF00268673. [DOI] [PubMed] [Google Scholar]
- Kiesewetter S., Fischer W., Sprinzl M. Sequences of three minor tRNAsArg from E. coli. Nucleic Acids Res. 1987 Apr 10;15(7):3184–3184. doi: 10.1093/nar/15.7.3184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K., Favre M. Maturation of the head of bacteriophage T4. I. DNA packaging events. J Mol Biol. 1973 Nov 15;80(4):575–599. doi: 10.1016/0022-2836(73)90198-8. [DOI] [PubMed] [Google Scholar]
- Lindahl L., Zengel J. M. Ribosomal genes in Escherichia coli. Annu Rev Genet. 1986;20:297–326. doi: 10.1146/annurev.ge.20.120186.001501. [DOI] [PubMed] [Google Scholar]
- Lupski J. R., Godson G. N. The rpsU-dnaG-rpoD macromolecular synthesis operon of E. coli. Cell. 1984 Dec;39(2 Pt 1):251–252. doi: 10.1016/0092-8674(84)90001-1. [DOI] [PubMed] [Google Scholar]
- McCarthy J. E., Schairer H. U., Sebald W. Translational initiation frequency of atp genes from Escherichia coli: identification of an intercistronic sequence that enhances translation. EMBO J. 1985 Feb;4(2):519–526. doi: 10.1002/j.1460-2075.1985.tb03659.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKeever W. G., Neidhardt F. C. Growth rate modulation of four aminoacyl-transfer ribonucleic acid synthetases in enteric bacteria. J Bacteriol. 1976 May;126(2):634–645. doi: 10.1128/jb.126.2.634-645.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neidhardt F. C., Bloch P. L., Pedersen S., Reeh S. Chemical measurement of steady-state levels of ten aminoacyl-transfer ribonucleic acid synthetases in Escherichia coli. J Bacteriol. 1977 Jan;129(1):378–387. doi: 10.1128/jb.129.1.378-387.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neidhardt F. C., Bloch P. L., Smith D. F. Culture medium for enterobacteria. J Bacteriol. 1974 Sep;119(3):736–747. doi: 10.1128/jb.119.3.736-747.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura M., Gourse R., Baughman G. Regulation of the synthesis of ribosomes and ribosomal components. Annu Rev Biochem. 1984;53:75–117. doi: 10.1146/annurev.bi.53.070184.000451. [DOI] [PubMed] [Google Scholar]
- Ny T., Björk G. R. Growth rate-dependent regulation of transfer ribonucleic acid (5-methyluridine) methyltransferase in Escherichia coli B/r. J Bacteriol. 1980 Jan;141(1):67–73. doi: 10.1128/jb.141.1.67-73.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ny T., Thomale J., Hjalmarsson K., Nass G., Björk G. R. Non-coordinate regulation of enzymes involved in transfer RNA metabolism in Escherichia coli. Biochim Biophys Acta. 1980 Apr 30;607(2):277–284. doi: 10.1016/0005-2787(80)90080-5. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Pedersen S., Bloch P. L., Reeh S., Neidhardt F. C. Patterns of protein synthesis in E. coli: a catalog of the amount of 140 individual proteins at different growth rates. Cell. 1978 May;14(1):179–190. doi: 10.1016/0092-8674(78)90312-4. [DOI] [PubMed] [Google Scholar]
- Petersen H. U., Joseph E., Ullmann A., Danchin A. Formylation of initiator tRNA methionine in procaryotic protein synthesis: in vivo polarity in lactose operon expression. J Bacteriol. 1978 Aug;135(2):453–459. doi: 10.1128/jb.135.2.453-459.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowen L., Kornberg A. Primase, the dnaG protein of Escherichia coli. An enzyme which starts DNA chains. J Biol Chem. 1978 Feb 10;253(3):758–764. [PubMed] [Google Scholar]
- Schnier J., Kitakawa M., Isono K. The nucleotide sequence of an Escherichia coli chromosomal region containing the genes for ribosomal proteins S6, S18, L9 and an open reading frame. Mol Gen Genet. 1986 Jul;204(1):126–132. doi: 10.1007/BF00330199. [DOI] [PubMed] [Google Scholar]
- Sharp P. M., Li W. H. The codon Adaptation Index--a measure of directional synonymous codon usage bias, and its potential applications. Nucleic Acids Res. 1987 Feb 11;15(3):1281–1295. doi: 10.1093/nar/15.3.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprinzl M., Hartmann T., Meissner F., Moll J., Vorderwülbecke T. Compilation of tRNA sequences and sequences of tRNA genes. Nucleic Acids Res. 1987;15 (Suppl):r53–188. doi: 10.1093/nar/15.suppl.r53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas M. S., Bedwell D. M., Nomura M. Regulation of alpha operon gene expression in Escherichia coli. A novel form of translational coupling. J Mol Biol. 1987 Jul 20;196(2):333–345. doi: 10.1016/0022-2836(87)90694-2. [DOI] [PubMed] [Google Scholar]
- Yokota T., Sugisaki H., Takanami M., Kaziro Y. The nucleotide sequence of the cloned tufA gene of Escherichia coli. Gene. 1980 Dec;12(1-2):25–31. doi: 10.1016/0378-1119(80)90012-8. [DOI] [PubMed] [Google Scholar]




