Abstract
Dimorphic growth of the budding yeast Saccharomyces cerevisiae is regulated by the quality of the nitrogen supply. On a preferred nitrogen source diploid cells grow as ellipsoidal cells by using a bipolar pattern of budding, whereas on a poor nitrogen source a unipolar pattern of budding is adopted, resulting in extended pseudohyphal chains of filamentous cells. Here we report that the quality of the nitrogen source is signaled by the glutamine tRNA isoform with a 5′-CUG anticodon (tRNACUG). Mutations that alter this tRNA impair assessment of the nitrogen supply without measurably affecting protein synthesis, so that mutant cells display pseudohyphal growth even on a preferred nitrogen source. The nitrogen status for other nitrogen-responsive processes such as catabolic gene expression and sporulation also is signaled by this tRNA: mutant cells inappropriately induce the nitrogen-repressed gene CAR1 and undergo precocious sporulation in nitrogen-rich media. Therefore, in addition to its role in mRNA translation, this tRNA also transduces nitrogen signals that regulate development.
All cells have sensitive mechanisms to sense and respond to their environment, including the availability of nutrients. For the budding yeast Saccharomyces cerevisiae, the “quality” of the nutritional environment regulates several developmental processes. For example, the process of meiosis and sporulation of diploid yeast cells is regulated in part by the nitrogen source, in that many sources of nitrogen can prevent meiosis and sporulation (reviewed in ref. 1). The morphological growth alternative of pseudohyphal development by diploid cells also is regulated by the nitrogen source: pseudohyphal growth is permitted by a poor nitrogen source such as proline, but prevented by a preferred nitrogen source such as ammonium ions (2). For each of these developmental alternatives, signaling networks that mediate the proper execution of these processes are being identified (1, 3, 4), and for pseudohyphal growth a regulatory component has been found with the potential for nitrogen sensing (5). However, for neither pseudohyphal growth nor sporulation is the mechanism(s) for sensing and/or initial signaling of the availability or quality of the nitrogen source well understood.
Here we report that the nitrogen-responsive developmental processes of pseudohyphal growth and sporulation are both affected by a yeast tRNA molecule. We show that mutations that affect the sequence of this tRNA, which decodes the codon CAG using the anticodon CUG, can bring about pseudohyphal growth without measurable effects on overall protein synthesis and, specifically, without effect on the decoding of CAG codons. This pseudohyphal growth of mutant cells takes place in nitrogen-rich conditions that inhibit the pseudohyphal growth of wild-type cells. The same tRNACUG mutations allow sporulation in nitrogen-rich media and derepress transcription of the nitrogen-repressed gene CAR1. The tRNACUG molecule is aminoacylated with glutamine, suggesting that glutaminyl-tRNACUG level is a signal of nitrogen status for the regulation of pseudohyphal growth and sporulation.
MATERIALS AND METHODS
Strains and Growth Conditions.
S. cerevisiae wild-type strain 21R (MATa ade1–1 ura3–52 leu2–3,112) has been described (6). Multiple backcrosses to strain 21R created diploid strains LMD651U (MATa/MATα sup70–65/sup70–65 ura3–52/ura3–52 leu2–3,112/LEU2 ade1–1/ADE1), LMD651WLU (MATa/MATα sup70–65/SUP70 ura3–52/ura3–52 leu2–3,112/leu2–3,112 ade1–1/ADE1 his3–11/HIS3 trp1–1/TRP1), LMDWU (MATa/MATα SUP70/SUP70 ura3–52/ura3–52 leu2–3,112/LEU2 ade1–1/ADE1), 9A6D (MATa/MATα ura3–52/ura3–52 leu2–3,112/LEU2 ade1–1/ADE1 his3–11/HIS3 trp1–1/trp1–1), and 6565D (MATa/MATα sup70–65/sup70–65 ade2–1/ADE2 his6/HIS6 ura1/URA1 ura3–52/URA3) and haploid strains JP65–1 (MATa sup70–65 ade2–1 ura3–52 leu2–3 112) (ref. 5) and NR65–33 (sup70–33 ade6 ura3–52). Gene disruption of STE20, STE11, STE7, and STE12 was by directed integration of plasmids pEL45 (ref. 7; from M. Whiteway, Biotechnology Research Institute, Montreal), pSL1311, pSL1077, and pSL1094 (ref. 8; from D. Thomas, Biotechnology Research Institute, Montreal) into strain LMD651WLU. Segregants from these integrants were transformed with complementing plasmids and mated to construct, after plasmid loss, strains DS3D-9c (MATa/MATα ste20Δ∷URA3/ste20Δ∷URA3 sup70–65/sup70–65 ura3–52/ura3–52 leu2–3,112/leu2–3,112 his3–11/his3–11), MLD64 (MATa/MATα ste11Δ∷URA3/ste11Δ∷URA3 sup70–65/sup70–65 ura3–52/ura3–52 leu2–3,112/leu2–3,112 his3–11/HIS3 ade1/ADE1), MLD60 (MATa/MATα ste7Δ∷ URA3/ste7Δ∷URA3 sup70–65/sup70–65 ura3–52/ura3–52 leu2–3,112/leu2–3,112 ade1/ADE1), and MLD66 (MATa/MATα ste12Δ∷URA3/ste12Δ∷URA3 sup70–65/sup70–65 ura3–52/ura3–52 leu2–3,112/leu2–3,112 his3–11/HIS3 ade1/ADE1). Ochre suppression used strains MLD14 (MATa/MATα sup70–65/sup70–65 trp1–1/trp1–1 ura3–52/ura3–52 leu2–3,112/LEU2 his3–11/his3–11 ade1/ADE1), MLD15 (MATa/MATα sup70–65/SUP70 trp1–1/trp1–1 ura3–52/ura3–52 leu2–3,112/LEU2 his3–11/his3–11 ade1/ADE1), MLD13 (MATa/MATα sup70–65/SUP70 trp1–1/TRP1 ura3–52/ura3–52 leu2–3,112/leu2–3,112 his3–11/his3–11 ade1/ADE1), and MLD17 (MATa/MATα trp1–1/trp1–1 ura3–52/ura3–52 his3–11/his3–11 ade1/ade1). The Σ1278b S. cerevisiae strains 646–11b (MATa gln4–1) and 402–5b (MATα gln1–105 his4 lys23), provided by A. Mitchell (9), were used to construct strains LGD11–17 (MATa/MATα gln4–1/gln4–1 gln1–105/gln1–105 his4/HIS4 lys23/LYS23) and LGD16–16 (MATa/MATα GLN4/GLN4 GLN1/GLN1 his4/HIS4 lys23/LYS23); the gln1–105 mutation enhances the gln4–1 phenotype (A. Mitchell, personal communication). Escherichia coli strains TOP10 (Invitrogen) and DH5αF′ (ref. 10) were used for plasmid amplification and growth of M13, respectively. Cells were grown on standard solid or liquid medium (11), and routine yeast genetic procedures were employed for strain construction (11).
tk;2Cloning Alleles of SUP70.
The oligonucleotides 5′-aatggatccTACCAGTACAAGATCCATTG (BamHI site) and 5′-taactgcaGAGCGATTGTATCTGTATAG (PstI site) were used as PCR primers to amplify 311-bp genomic DNA fragments encoding SUP70, sup70–65, and sup70–33 from wild-type and mutant strains. Amplification (12) generated BamHI and PstI sites at opposite termini to facilitate cloning into M13 mp18 (ref. 13) for DNA sequence determination.
Chimeric tRNA Genes.
DNA fragments were eluted from 12% polyacrylamide gels by diffusion (14). The 91-bp MseI-Sau3AI fragment containing the 5′ end and 5′-CTG anticodon of SUP70 and the 93-bp Sau3AI-NheI fragment containing the 3′ end of SRM2 (ref. 15) were ligated to NdeI/XbaI-cleaved pUC19 to generate pLM6. The 73-bp HincII-Sau3AI fragment containing the 5′ end and 5′-TTG anticodon of SRM2 and the 65-bp Sau3AI/MseI fragment containing the 3′ end of SUP70 were ligated to HincII/NdeI-cleaved pUC19 to generate pLM9. The chimeric tRNA genes were excised from pLM6 and pLM9 by cleavage with AvaII and used to replace the PpuMI fragment of SUP70 in p4C3, which bears SUP70 in its chromosomal context, generating pLM11 and pLM12, respectively. Orientation and sequence of these chimeric genes were confirmed by nucleotide sequencing. The chimeric tRNA genes and flanking sequences were excised from pLM11 and pLM12 as EcoRI-BamHI fragments and ligated into EcoRI/BamHI-cleaved pRS316 (ref. 16) to generate pAW19 and pAW20, respectively.
Plasmids to Evaluate Translation Efficiency.
Two glutamine-encoding oligonucleotide cassettes were ligated to the hybrid yeast CYC1/E. coli lacIZ reporter gene in plasmid pLG669Z-Δ229–176 (ref. 17). Translation of these modified lacZ genes adds repeats of Ser-(Gln)5-Arg at the amino terminus of the β-galactosidase protein. An insertion cassette containing 5′-CAG glutamine codons was used to create the pQG plasmid set, and a cassette containing 5′-CAA glutamine codons was used for the pQA set. To generate these cassettes, the oligonucleotide pairs 5′-GATCACAGCAGCAGCAGCAGA and 5′-GATCTCTGCTGCTGCTGCTGT, or 5′-GATCACAACAACAACAACAAA and 5′-GATCTTTGTTGTTGTTGTTGT were annealed and ligated to form concatemers that then were resolved electrophoretically by using polyacrylamide. DNA from bands containing monomers and multimers were isolated separately (14) and ligated to BamHI-cleaved pLG669Z-Δ229–176, and the ligation products were cleaved with BamHI + BclI + BglII to linearize religated vector and remove concatemers ligated in incorrect orientation. Number and orientation of inserted cassettes were determined by sequencing using the primer 5′-CATTAGGTCCTTTGTAGC. β-galactosidase assays were as described (18), except that cells were broken by vortexing samples containing glass beads. Protein concentrations were determined by using a Bio-Rad assay kit.
RNA Blots.
CAR1 mRNA was detected with the 0.89-kbp BglII fragment of plasmid pRS11 (ref. 19), ACT1 mRNA was detected with a 1-kbp HindIII-XhoI fragment from pRS208 (from R. Storms, Concordia University, Montreal), and lacZ mRNA was detected with the 3.1-kbp BamHI-DraI fragment of YIp102 (ref. 20). Signals were quantified by scanning densitometry of autoradiograms and normalized to ACT1 mRNA levels.
RESULTS
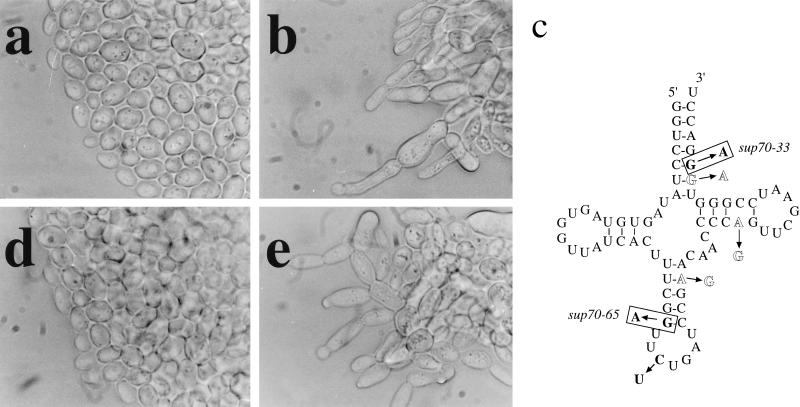
Dimorphic growth of the budding yeast Saccharomyces cerevisiae is regulated by the quality of the nitrogen supply. On a preferred nitrogen source diploid cells grow as ellipsoidal cells by using a bipolar pattern of budding (21, 22). For diploid cells growing on solid medium this bipolar budding results in the production of a colony with a smooth edge (Fig. 1a). In contrast, cells proliferating on a poor nitrogen source adopt a unipolar budding pattern; on solid medium this budding pattern leads to the formation of pseudohyphae, and colonies are formed with chains of cells extending from the colony edge (2). We found that two mutations in the CDC65 gene (23) cause diploid cells to form pseudohyphae even on a preferred nitrogen source (Fig. 1b).
Figure 1.
tRNACUG alterations and anticodon change impair signaling. (a and b) Mutant cells exhibit pseudohyphal growth. Diploid wild-type (strain LMDWU) (a) or sup70–65 mutant cells (strain LMD651U) (b) were incubated on nitrogen-rich solid complex medium. (c) tRNACUG, with sequence changes in sup70–65 and sup70–33 boxed, the tRNAUUG anticodon change in bold, and other tRNAUUG changes in outline. (d and e) The CUG anticodon specifies nitrogen signaling. sup70–65 mutant cells (strain LMD651U) containing plasmid-encoded chimeric tRNAUUG with the anticodon 5′-CUG (d) or chimeric tRNACUG with the anticodon 5′-UUG (e) were incubated on nitrogen-rich selective medium.
tRNACUG Affects Pseudohyphal Growth.
The CDC65 gene was cloned by complementation (23), subcloned, and sequenced, revealing that the gene product is a tRNA with a 5′-CUG anticodon, which recognizes the codon CAG specifying glutamine. This tRNA gene has been described previously (24–26) and named SUP70 (ref. 26); henceforth we use this name and designate the mutant alleles sup70–65 and sup70–33. Genome analysis shows that SUP70 is the only gene encoding tRNACUG. SUP70 is an essential gene (25), and we found that cells in the microcolonies formed by meiotic segregants lacking SUP70 exhibited pseudohyphal growth (data not shown). The pseudohyphal morphology displayed by sup70–65 diploid cells was prevented by the presence of a low-copy plasmid carrying the wild-type SUP70 gene, and heterozygous (SUP70/sup70–65) diploid cells were indistinguishable from wild-type cells (data not shown). The sup70–65 mutation therefore is recessive for pseudohyphal growth, indicating that the sup70–65 mutation impairs a function of tRNACUG that mediates appropriate responses to preferred nitrogen sources.
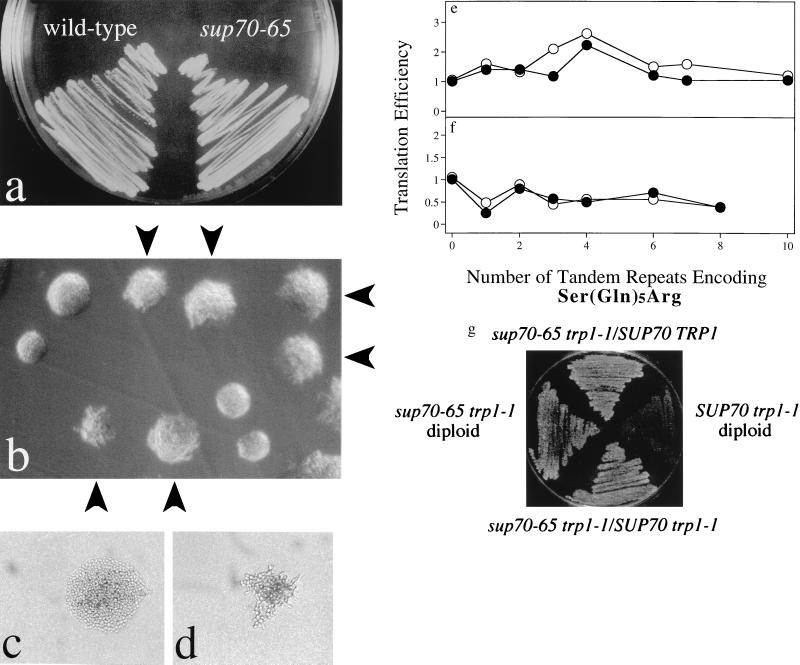
The sup70 mutant alleles that we identified were cloned by amplification of genomic DNA from mutant cells by using PCR. DNA sequencing revealed that each mutant tRNA contains a different single-nucleotide change affecting intramolecular base pairing (Fig. 1c). Thus, secondary and tertiary structure of this tRNA are important for the regulation of pseudohyphal growth. This conclusion is consistent with the finding that pseudohyphal growth is displayed by sup70–65 and sup70–33 mutant cells at 30°C and 34°C, respectively, whereas at 23°C, normal cell morphology and budding patterns are restored (data not shown). Modification or interactions of these altered tRNAs therefore may be affected by temperature. However, any secondary or tertiary structural changes of this tRNA do not affect its ability to interact with its aminoacylating enzyme or with the translation machinery, for the sup70–65 tRNA provides effective ochre suppression (27) during mRNA translation at both 30°C and 23°C (Fig. 2g).
Figure 2.
Normal translation in sup70–65 cells. (a–d) Isogenic diploid wild-type (strain LMDWU) and sup70–65 mutant cells (strain LMD651U) were incubated on nitrogen-rich solid complex medium at 30°C. Arrows in b indicate sup70–65 mutant colonies; unmarked colonies are wild type. In c and d the medium contained cycloheximide to slow the growth of wild-type (c) and mutant (d) cells by 50%. (e and f) CAG codons are translated efficiently in sup70–65 mutant cells. β-Galactosidase activity was measured for extracts made from sup70–65 (strain LMD651U) (closed symbols) and wild-type (strain LMDWU) cells (open symbols) proliferating at 30°C and harboring plasmids pQA1, pQA2, pQA3, pQA4, pQA6, pQA7, pQA10, pQG1, pQG2, pQG3, pQG4, pQG6, or pQG8. The plasmid-encoded β-galactosidase contains (Ser-Gln5-Arg)n inserted near the N terminus, with n indicated by the number in the plasmid name. Inserted glutamines all are encoded by CAG for pQG plasmids (e) and by CAA for pQA plasmids (f). Assays were performed in duplicate, and values normalized to protein content, expressed in Miller units (×103), were used as a measure of translation efficiency. (g) Ochre suppression. Diploid strains (MLD14, MLD15, MLD17, and MLD13) with the indicated genotypes were incubated at 30°C on tryptophan-free solid medium.
Three nucleotide differences outside the anticodon distinguish tRNACUG from tRNAUUG, the other yeast glutamine tRNA (Fig. 1c). Using a cloned tRNAUUG gene we constructed a pair of chimeric genes encoding tRNAs with the anticodon of one glutamine tRNA and the backbone of the other. These chimeric tRNA genes were cloned into a plasmid-borne SUP70 genomic locus to maintain the SUP70 chromosomal context and transformed into sup70–65 mutant cells. As shown in Fig. 1d, mutant cells expressing the chimeric tRNA with the backbone of tRNAUUG but the 5′-CUG anticodon grew without pseudohyphae, whereas mutant cells expressing the version of SUP70 tRNA with the 5′-UUG anticodon remained unable to signal nitrogen status and exhibited pseudohyphal growth (Fig. 1e). The CUG anticodon thus is a critical component for proper nitrogen signaling by this tRNA.
Efficient Translation of CAG Codons During Pseudohyphal Growth.
During the pseudohyphal growth by diploid mutant cells there is effective use of available nitrogen for biosynthetic purposes: sup70–65 mutant cells were unaffected for growth and colony formation on solid nitrogen-rich medium (Fig. 2 a and b) and proliferated in liquid nitrogen-rich medium at rates indistinguishable from those of wild-type cells (data not shown). Therefore, the sup70–65 tRNACUG molecule recognizes the CAG codon during mRNA translation. Another indication that tRNACUG does not affect pseudohyphal growth through effects on translation is the finding that conditions that limit global protein synthesis do not bring about pseudohyphal development. Treatment of cells either with cycloheximide to impair translation elongation or with 3-aminotriazole to inhibit histidine biosynthesis, at concentrations that, in each case, slowed the rate of cell proliferation by 50%, failed to induce pseudohyphal growth (Fig. 2c and data not shown). Under these limiting conditions sup70–65 mutant cells still displayed pseudohyphal growth at 30°C but not at 23°C (Fig. 2d and data not shown). Thus, tRNACUG does not influence pseudohyphal development through global effects on translation; sup70 mutant cells use nitrogenous resources efficiently for protein synthesis but are unable to signal the quality of the nitrogen supply for morphogenetic regulation.
For a more direct assessment of CAG codon recognition during mRNA translation we constructed reporter genes encoding β-galactosidase variants with inserted tracts of glutamine specified by either CAG or CAA, the other glutamine codon. These reporter genes then were expressed in sup70–65 mutant cells. The added coding sequences had little effect on mRNA abundance in these cells (data not shown). Similarly, mutant and wild-type cells contained equal amounts of enzyme activity from each glutamine-enriched β-galactosidase variant, regardless of whether the additional glutamines were encoded by CAG or CAA codons (Fig. 2 e and f). Cells with the altered tRNACUG translated mRNAs containing up to 40 closely spaced CAG codons as efficiently as did wild-type cells. This result is consistent with the normal growth rate of sup70–65 mutant cells and indicates that the altered tRNACUG in these cells does not compromise the translation of CAG codons. The absence of translation effects, even for CAG-enriched mRNAs, suggests that nitrogen signaling by tRNACUG is not transmitted through new protein synthesis. tRNACUG most likely regulates a signaling pathway communicating nitrogen status for dimorphic growth.
The Ste MAP-Kinase Pathway and the Ste12 Transcription Factor Are Dispensable for Pseudohyphal Growth by tRNACUG Mutants.
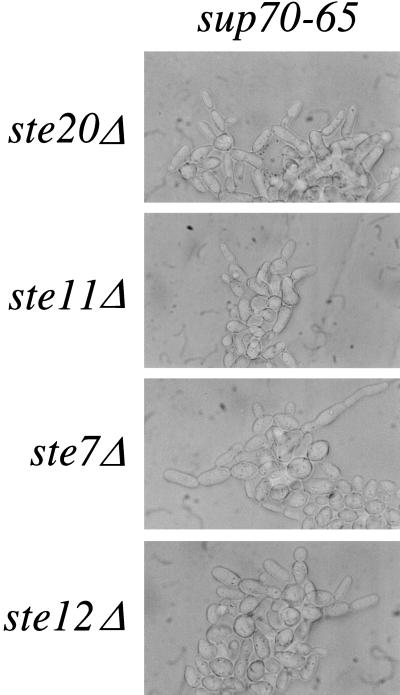
Pseudohyphal growth is facilitated by a signal-transduction pathway that includes the Ste20, Ste11, and Ste7 protein kinases and the Ste12 transcription factor: inactivation of any of these proteins compromises the pseudohyphal growth of SUP70 cells (3, 4). However, diploid cells homozygous for sup70–65 and for ste20Δ, ste11Δ, ste7Δ, or ste12Δ mutations still exhibited pseudohyphal growth (Fig. 3), indicating that tRNACUG signals nitrogen status independently of this pathway.
Figure 3.
tRNACUG effects are independent of the Ste signal-transduction pathway. Diploid strains homozygous for sup70–65 and the indicated ste deletion were incubated on nitrogen-rich solid complex medium at 30°C.
tRNACUG Inhibits Nitrogen Repression.
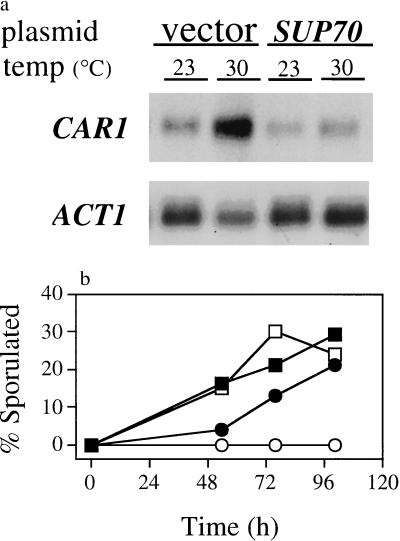
To assess tRNACUG signaling in other nitrogen-responsive activities we examined nitrogen-regulated gene expression. Expression of the CAR1 gene is repressed strongly by NH4+ (ref. 28) and increases in cells deprived of this preferred nitrogen source (19, 29). For sup70–65 mutant diploid cells growing with NH4+ as the nitrogen source at 23°C, a temperature at which signaling for morphogenesis is adequate to prevent pseudohyphal growth, CAR1 mRNA levels were equivalent to those for wild-type cells. After sup70–65 mutant cells were transferred to 30°C CAR1 mRNA levels increased by approximately 2- to 4-fold with respect to CAR1 mRNA abundance in mutant cells made phenotypically wild type by the presence of a plasmid-borne SUP70 gene (Fig. 4a). Thus, CAR1 repression by the preferred nitrogen source NH4+ is also mediated by tRNACUG signaling. On the other hand, the hyperexpression of CAR1 that occurs when arginine is the nitrogen source (28) was unaffected in sup70–65 mutant cells (data not shown). Thus, only certain types of nitrogen-related signaling involve this tRNA.
Figure 4.
sup70–65 impairs nitrogen signaling for gene expression and sporulation. (a) CAR1 gene expression. RNA was extracted from sup70–65 mutant cells (strain LMD651U) growing in nitrogen-rich complex medium at 23°C or after 1 h of further incubation at 30°C, resolved, blotted, and probed for CAR1 mRNA and for ACT1 mRNA as a loading control. Vector lanes, cells harboring control vector YEp352; SUP70 lanes, cells harboring multicopy SUP70 plasmid pS4B-21. (b) Sporulation. Wild-type (strain 9A6D) (open symbols) and sup70–65 mutant cells (strain 6565D) (closed symbols) were incubated at 30°C on solid nitrogen-rich YEPD medium, then replica-plated to nitrogen-rich YEPG medium (circles) or to nitrogen-free sporulation medium (squares) as positive controls. Cell samples were suspended in water, and sporulation was determined visually; at least 100 cells were evaluated per sample.
tRNACUG Inhibits Meiosis and Sporulation.
The meiosis and subsequent sporulation of diploid cells is another developmental process responsive to nitrogen status. Diploid cells can undergo sporulation when starved for nitrogen during growth on a nonfermentable carbon source such as glycerol, whereas NH4+ prevents sporulation (30). In contrast, diploid sup70–65 mutant cells sporulated at 30°C on rich (YEPG) medium (11) that contained enough nitrogenous material to prevent the sporulation of congenic wild-type diploid cells (Fig. 4b). As with pseudohyphal growth, this precocious sporulation is a recessive trait: diploid cells heterozygous for sup70–65 did not sporulate under these conditions. Therefore, tRNACUG signals nitrogen status to inhibit meiosis and sporulation as well as pseudohyphal growth.
A fermentable carbon source such as glucose prevents sporulation by wild-type cells (31). The precocious sporulation of diploid sup70–65 mutant cells was prevented by the presence of glucose (data not shown). Thus, the SUP70 tRNACUG is not involved in discrimination of carbon source and may be restricted to nitrogen signaling.
DISCUSSION
We report here that alterations in the structure of a yeast tRNA molecule can affect the ability of the cell to respond properly to its environment. We find that yeast cells harboring mutant forms of tRNACUG display several responses that normally are repressed by a preferred nitrogen source. The mutant cells undergo pseudohyphal development, derepress transcription of the nitrogen-repressed gene CAR1, and undertake precocious meiosis and sporulation by circumventing the nitrogen-mediated repression of these processes.
The tRNA mutations affect the only gene in these cells that encodes tRNACUG, the molecule that decodes the glutamine codon CAG. It is clear that these structurally altered tRNACUG molecules function effectively in mRNA translation: mutant cells grow at normal rates and are unaffected in the ability to translate reporter mRNAs specifically enriched for up to 40 closely spaced CAG codons. These observations indicate that the mutant tRNA molecules interact efficiently with the glutaminyl-tRNA synthetase charging enzyme and with the translation machinery. Nevertheless, these mutant tRNA molecules compromise the proper signaling of the quality of the nitrogen supply. The wild-type tRNACUG molecule therefore is necessary for this signaling function.
We find that several components that facilitate pseudohyphal growth, including the “Ste” MAP-kinase signaling pathway and the Ste12 transcription factor (3, 4), are not necessary for the pseudohyphal growth of mutant cells with altered tRNACUG. Recently, another component that affects pseudohyphal growth, the high-affinity ammonium permease Mep2, also has been shown to be independent of this MAP-kinase pathway (5). The effects of mep2 and sup70 mutations reveal distinct roles for these gene products in nitrogen signaling, and, at present, the functional relationship between the Mep2 permease and tRNACUG remains to be determined.
To account for the involvement of a tRNA molecule in signaling we favor a model in which nitrogen signaling is mediated by the interaction of glutaminyl-tRNACUG with a regulatory protein. Glutamine is a central intermediate in nitrogen metabolism (32); glutaminyl-tRNACUG thus could be a sensitive transducer of nitrogen status. The uncharged form of tRNACUG may not participate in nitrogen signaling, because diploid SUP70 cells with impaired tRNACUG aminoacylation from the gln4–1 mutation [affecting glutaminyl-tRNA synthetase (9, 33)] grew poorly but did not exhibit pseudohyphal growth (unpublished observation). The recessive nature of the sup70–65 mutation for nitrogen signaling suggests that interaction of tRNACUG with a regulatory protein is necessary for the maintenance of proper signaling, whereas the effective decoding of CAG codons in mutant cells indicates that this signaling interaction in particular is affected by the sup70–65 and sup70–33 alterations of tRNACUG. Our demonstration that the CUG anticodon is important in nitrogen signaling suggests that signaling interactions may involve anticodon recognition. This model therefore proposes a central and nontranslational role for tRNACUG in intracellular signaling.
A nontranslational signaling role for tRNA has a precedent in the yeast system. For the general control of amino acid biosynthesis, tRNA molecules in the uncharged form interact with a regulatory domain of the Gcn2 protein kinase (34), which is thought to signal the availability of amino acids. This tRNA–protein interaction regulates Gcn2 protein kinase activity (34, 35), and is distinct from the tRNA interactions found during mRNA translation. Similar to the tRNA–Gcn2 situation, our model for nitrogen signaling proposes a specific interaction for tRNACUG with a regulatory protein that is in addition to the specific tRNA–protein interaction that already exists between tRNACUG and its aminoacylating enzyme.
Acknowledgments
We thank C. Barnes, T. Cooper, L. Guarente, P. Hieter, R. Storms, D. Thomas, and M. Whiteway for plasmids, A. Mitchell for strains and advice, and an anonymous reviewer for comments. We thank A. Wheeler Reich for construction of the pQG and pQA plasmids and for technical assistance, and K. Gillis, S. Harvie, and D. Carruthers for technical assistance. This work was supported by grants from the Medical Research Council of Canada (jointly to G.C.J. and R.A.S.) and the Australian Research Council (to I.W.D.). G.C.J. is a Terry Fox Cancer Research Scientist of the National Cancer Institute of Canada.
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
References
- 1.Kupiec M, Byers B, Esposito R E, Mitchell A P. In: The Molecular and Cellular Biology of the Yeast Saccharomyces. Pringle J R, Broach J R, Jones E W, editors. Vol. 3. Plainview, NY: Cold Spring Harbor Lab. Press; 1997. pp. 889–1036. [Google Scholar]
- 2.Gimeno C J, Ljungdahl P O, Styles C A, Fink G R. Cell. 1992;68:1077–1090. doi: 10.1016/0092-8674(92)90079-r. [DOI] [PubMed] [Google Scholar]
- 3.Liu H, Styles C A, Fink G R. Science. 1993;262:1741–1744. doi: 10.1126/science.8259520. [DOI] [PubMed] [Google Scholar]
- 4.Lo H-J, Köhler J R, DiDomenico B, Loebenberg D, Cacciapuoti A, Fink G R. Cell. 1997;90:939–949. doi: 10.1016/s0092-8674(00)80358-x. [DOI] [PubMed] [Google Scholar]
- 5.Lorenz M C, Heitman J. EMBO J. 1998;17:1236–1247. doi: 10.1093/emboj/17.5.1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rowley A, Singer R A, Johnston G C. Mol Cell Biol. 1991;11:5718–5726. doi: 10.1128/mcb.11.11.5718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leberer E, Dignard D, Harcus D, Thomas D Y, Whiteway M. EMBO J. 1992;11:4815–4824. doi: 10.1002/j.1460-2075.1992.tb05587.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stevenson B J, Rhodes N, Errede B, Sprague G F., Jr Genes Dev. 1992;6:1293–1304. doi: 10.1101/gad.6.7.1293. [DOI] [PubMed] [Google Scholar]
- 9.Mitchell A P, Ludmerer S W. J Bacteriol. 1984;158:530–534. doi: 10.1128/jb.158.2.530-534.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liss L R. Focus. 1986;8:9. [Google Scholar]
- 11.Guthrie, C. & Fink, G. R. (1991) Methods Enzymol. 194. [DOI] [PubMed]
- 12.Saluz H, Jost J-P. Proc Natl Acad Sci USA. 1989;86:2602–2606. doi: 10.1073/pnas.86.8.2602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Norrander J, Kempe T, Messing J. Gene. 1983;26:101–106. doi: 10.1016/0378-1119(83)90040-9. [DOI] [PubMed] [Google Scholar]
- 14.Smith H O. Methods Enzymol. 1980;65:371–380. doi: 10.1016/s0076-6879(80)65048-4. [DOI] [PubMed] [Google Scholar]
- 15.Boone C, Clark K L, Sprague G F., Jr Nucleic Acids Res. 1992;20:4461. doi: 10.1093/nar/20.17.4661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sikorski R S, Hieter P. Genetics. 1989;122:19–27. doi: 10.1093/genetics/122.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guarente L, Ptashne M. Proc Natl Acad Sci USA. 1981;78:2199–2203. doi: 10.1073/pnas.78.4.2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guarente L. Methods Enzymol. 1983;101:181–192. doi: 10.1016/0076-6879(83)01013-7. [DOI] [PubMed] [Google Scholar]
- 19.Sumrada R A, Cooper T G. Mol Cell Biol. 1982;2:1514–1523. doi: 10.1128/mcb.2.12.1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barnes C A, Johnston G C, Singer R A. Gene. 1991;104:47–54. doi: 10.1016/0378-1119(91)90463-l. [DOI] [PubMed] [Google Scholar]
- 21.Freifelder D. J Bacteriol. 1960;80:567–568. doi: 10.1128/jb.80.4.567-568.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hicks J B, Strathern J N, Herskowitz I. Genetics. 1977;85:395–405. doi: 10.1093/genetics/85.3.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Prendergast J A, Murray L E, Rowley A, Carruthers D R, Singer R A, Johnston G C. Genetics. 1990;124:81–90. doi: 10.1093/genetics/124.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Edelman I, Culbertson M R. EMBO J. 1991;10:1481–1491. doi: 10.1002/j.1460-2075.1991.tb07668.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weiss W A, Friedberg E C. J Mol Biol. 1986;192:725–735. doi: 10.1016/0022-2836(86)90024-0. [DOI] [PubMed] [Google Scholar]
- 26.Lin J P, Aker M, Sitney K C, Mortimer R K. Gene. 1986;49:383–388. doi: 10.1016/0378-1119(86)90375-6. [DOI] [PubMed] [Google Scholar]
- 27.Weiss W A, Edelman I, Culbertson M R, Friedberg E C. Proc Natl Acad Sci USA. 1987;84:8031–8034. doi: 10.1073/pnas.84.22.8031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Messenguy F, Dubois E. Mol Gen Genet. 1983;189:148–156. doi: 10.1007/BF00326068. [DOI] [PubMed] [Google Scholar]
- 29.Middelhoven W J. Biochim Biophys Acta. 1968;156:440–443. doi: 10.1016/0304-4165(68)90284-5. [DOI] [PubMed] [Google Scholar]
- 30.Miller J J. Can J Microbiol. 1963;9:259–277. [Google Scholar]
- 31.Miller J J. Can J Microbiol. 1957;3:81–90. doi: 10.1139/m57-010. [DOI] [PubMed] [Google Scholar]
- 32.Magasanik B. In: The Molecular and Cellular Biology of the Yeast Saccharomyces. Jones E W, Pringle J R, Broach J R, editors. Vol. 2. Plainview, NY: Cold Spring Harbor Lab. Press; 1992. pp. 283–317. [Google Scholar]
- 33.Ludmerer S W, Schimmel P. J Bacteriol. 1985;163:763–768. doi: 10.1128/jb.163.2.763-768.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wek S A, Zhu S, Wek R C. Mol Cell Biol. 1995;15:4497–4506. doi: 10.1128/mcb.15.8.4497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhu S, Sobolev A Y, Wek R C. J Biol Chem. 1996;271:24989–24994. doi: 10.1074/jbc.271.40.24989. [DOI] [PubMed] [Google Scholar]