Abstract
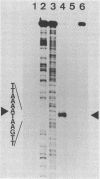
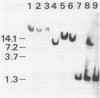
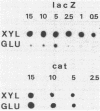
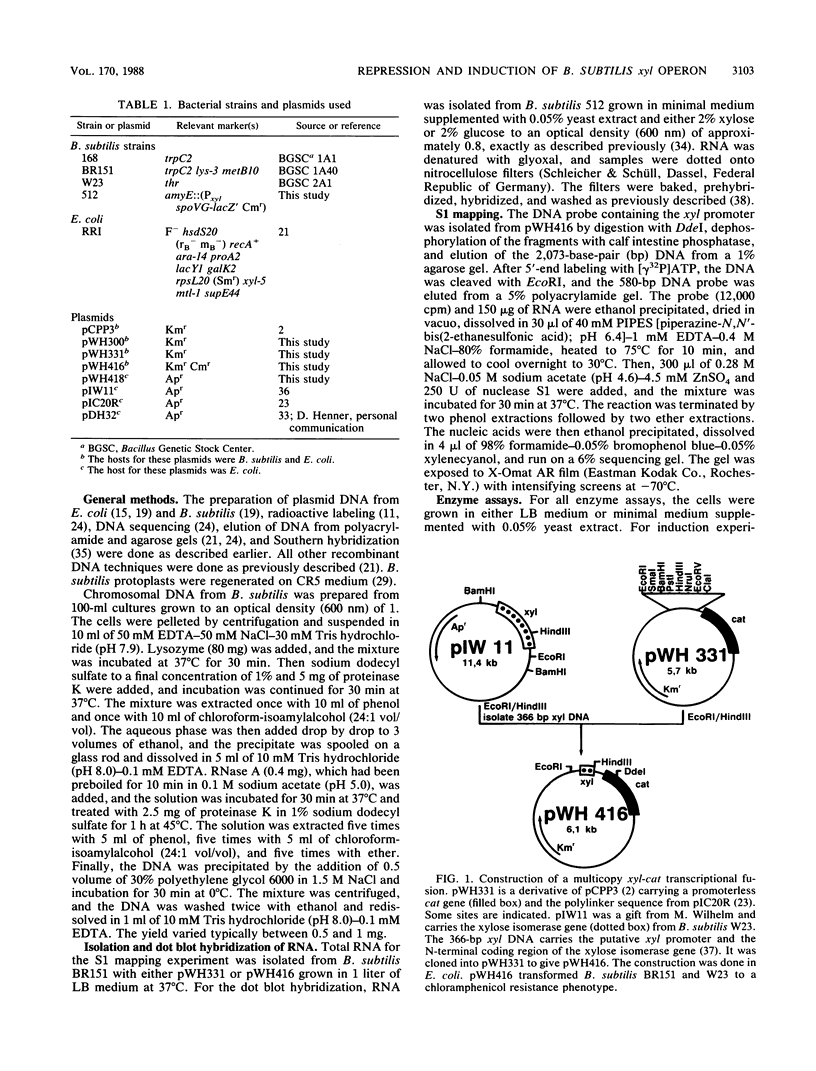
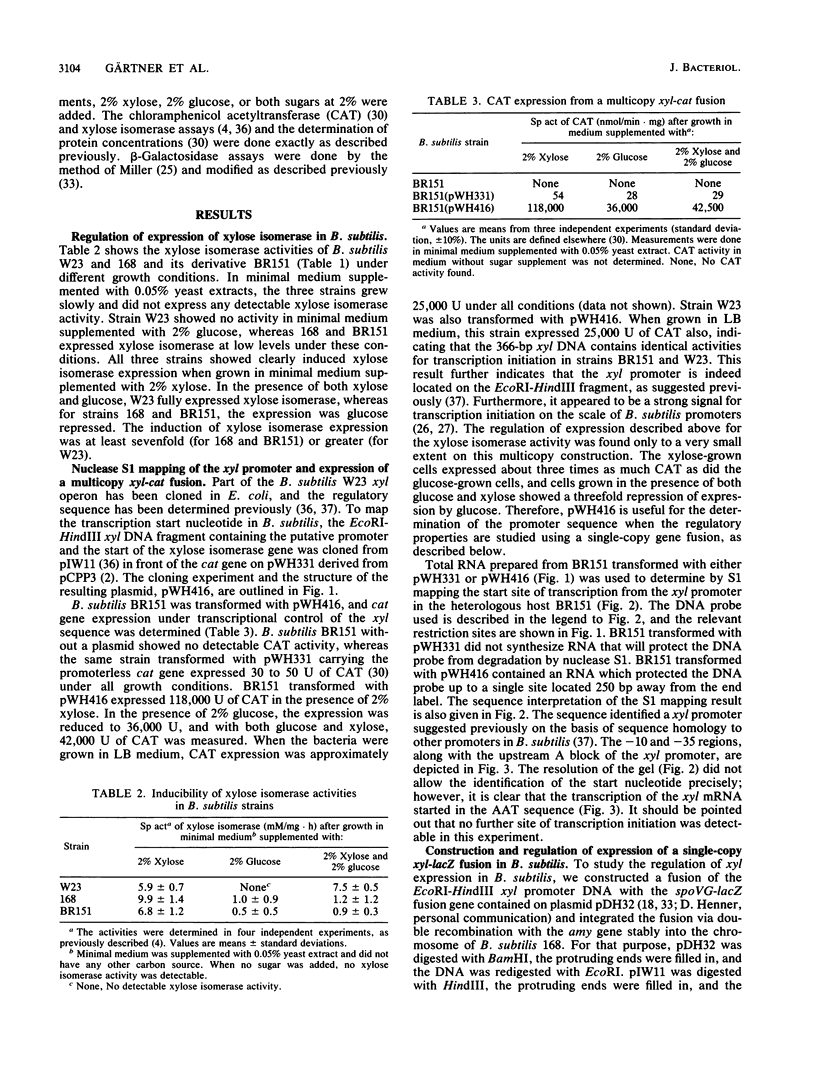
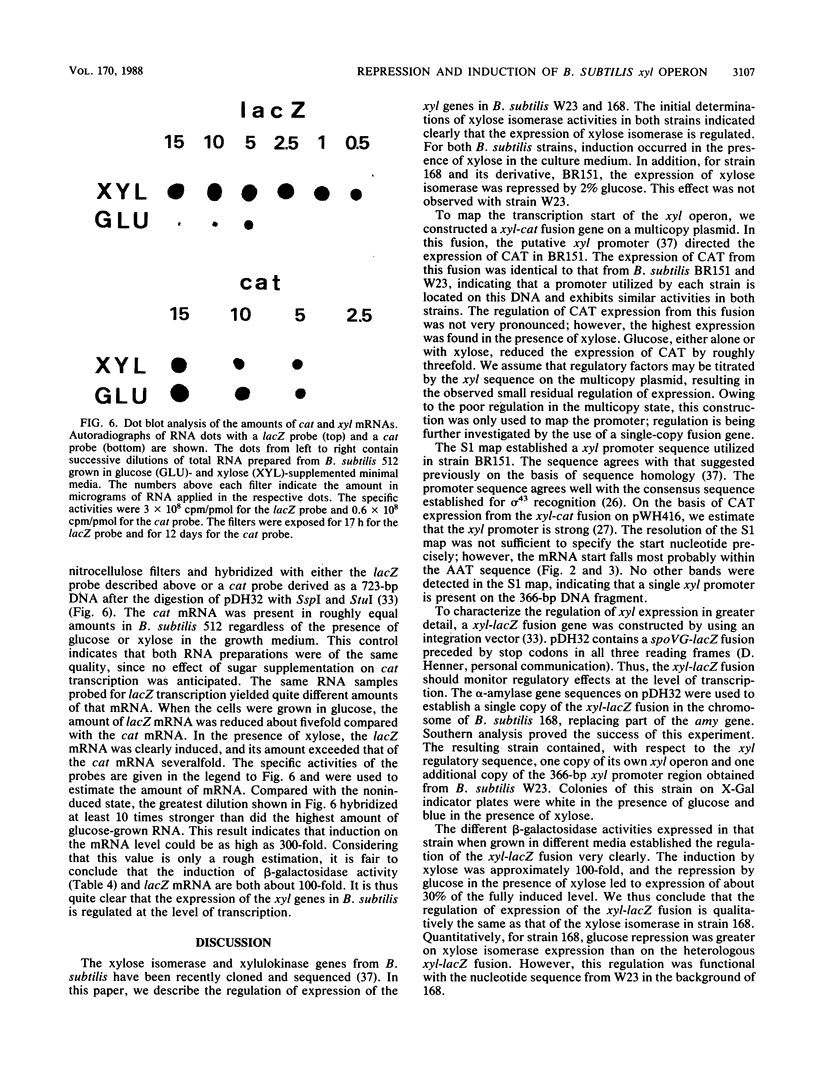
Expression of xylose isomerase was repressed in Bacillus subtilis strains W23, 168, and BR151 and could be induced in the presence of xylose. The expression was also glucose repressed in strains 168 and BR151, although this effect was not observed with W23. A xyl-cat fusion gene was constructed on a multicopy plasmid, from which the xyl promoter located on a 366-base-pair (bp) DNA fragment derived from W23 directed the expression of chloramphenicol resistance. The regulation of expression was not very pronounced in this multicopy situation. The xyl promoter is a strong signal for transcription initiation. The 5' sequence of the xyl mRNA was identified by nuclease S1 mapping. The promoter consisted of the -10 sequence TAAGAT, the -35 sequence TTGAAA spaced by 17 bp, and an upstream poly(A) block with 14 As out of 17 bp. To study the regulation, a xyl-lacZ fusion gene was constructed and integrated as a single copy into the amygene of B. subtilis 168. This strain grows blue on X-Gal (5-bromo-4-chloro-3-indolyl-beta-D-galactoside) indicator plates in the presence of xylose and white in the presence of glucose. Quantitatively, the induction of beta-galactosidase by xylose was 100-fold. In the presence of xylose plus glucose, the expression of the indicator gene was repressed to 30% of the fully induced level. About 25 to 60% of the maximal lacZ expression was obtained with this strain when the 366-bp xyl DNA fragment was provided in trans on a multicopy plasmid. This result indicates that repression in the absence of xylose is mediated in trans by a soluble factor which is expressed at a low level in B. subtilis 168. The xylose effect depended on negative regulation. The estimations of mRNA amounts by dot blot analysis showed unambiguously that the induction by xylose occurs at the level of transcription. The possible molecular mechanisms are discussed with respect to the nucleotide sequence of the 366-bp xyl regulatory DNA.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anagnostopoulos C., Spizizen J. REQUIREMENTS FOR TRANSFORMATION IN BACILLUS SUBTILIS. J Bacteriol. 1961 May;81(5):741–746. doi: 10.1128/jb.81.5.741-746.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Band L., Yansura D. G., Henner D. J. Construction of a vector for cloning promoters in Bacillus subtilis. Gene. 1983 Dec;26(2-3):313–315. doi: 10.1016/0378-1119(83)90204-4. [DOI] [PubMed] [Google Scholar]
- Briggs K. A., Lancashire W. E., Hartley B. S. Molecular cloning, DNA structure and expression of the Escherichia coli D-xylose isomerase. EMBO J. 1984 Mar;3(3):611–616. doi: 10.1002/j.1460-2075.1984.tb01856.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang S., Cohen S. N. High frequency transformation of Bacillus subtilis protoplasts by plasmid DNA. Mol Gen Genet. 1979 Jan 5;168(1):111–115. doi: 10.1007/BF00267940. [DOI] [PubMed] [Google Scholar]
- Cohen S. N., Chang A. C., Hsu L. Nonchromosomal antibiotic resistance in bacteria: genetic transformation of Escherichia coli by R-factor DNA. Proc Natl Acad Sci U S A. 1972 Aug;69(8):2110–2114. doi: 10.1073/pnas.69.8.2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David J. D., Wiesmeyer H. Control of xylose metabolism in Escherichia coli. Biochim Biophys Acta. 1970 Mar 24;201(3):497–499. doi: 10.1016/0304-4165(70)90171-6. [DOI] [PubMed] [Google Scholar]
- Davis E. O., Henderson P. J. The cloning and DNA sequence of the gene xylE for xylose-proton symport in Escherichia coli K12. J Biol Chem. 1987 Oct 15;262(29):13928–13932. [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Fisher S. H., Magasanik B. Isolation of Bacillus subtilis mutants pleiotropically insensitive to glucose catabolite repression. J Bacteriol. 1984 Mar;157(3):942–944. doi: 10.1128/jb.157.3.942-944.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita Y., Fujita T. Identification and nucleotide sequence of the promoter region of the Bacillus subtilis gluconate operon. Nucleic Acids Res. 1986 Feb 11;14(3):1237–1252. doi: 10.1093/nar/14.3.1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghangas G. S., Wilson D. B. Isolation and characterization of the Salmonella typhimurium LT2 xylose regulon. J Bacteriol. 1984 Jan;157(1):158–164. doi: 10.1128/jb.157.1.158-164.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillen W., Klein R. D., Wells R. D. Preparation of milligram amounts of 21 deoxyribonucleic acid restriction fragments. Biochemistry. 1981 Jun 23;20(13):3748–3756. doi: 10.1021/bi00516a013. [DOI] [PubMed] [Google Scholar]
- Himeno T., Imanaka T., Aiba S. Nucleotide sequence of the penicillinase repressor gene penI of Bacillus licheniformis and regulation of penP and penI by the repressor. J Bacteriol. 1986 Dec;168(3):1128–1132. doi: 10.1128/jb.168.3.1128-1132.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igo M. M., Losick R. Regulation of a promoter that is utilized by minor forms of RNA polymerase holoenzyme in Bacillus subtilis. J Mol Biol. 1986 Oct 20;191(4):615–624. doi: 10.1016/0022-2836(86)90449-3. [DOI] [PubMed] [Google Scholar]
- Klein R. D., Selsing E., Wells R. D. A rapid microscale technique for isolation of recombinant plasmid DNA suitable for restriction enzyme analysis. Plasmid. 1980 Jan;3(1):88–91. doi: 10.1016/s0147-619x(80)90037-2. [DOI] [PubMed] [Google Scholar]
- Marsh J. L., Erfle M., Wykes E. J. The pIC plasmid and phage vectors with versatile cloning sites for recombinant selection by insertional inactivation. Gene. 1984 Dec;32(3):481–485. doi: 10.1016/0378-1119(84)90022-2. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Moran C. P., Jr, Lang N., LeGrice S. F., Lee G., Stephens M., Sonenshein A. L., Pero J., Losick R. Nucleotide sequences that signal the initiation of transcription and translation in Bacillus subtilis. Mol Gen Genet. 1982;186(3):339–346. doi: 10.1007/BF00729452. [DOI] [PubMed] [Google Scholar]
- Osburne M. S., Craig R. J. Activity of two strong promoters cloned into Bacillus subtilis. J Gen Microbiol. 1986 Feb;132(2):565–568. doi: 10.1099/00221287-132-2-565. [DOI] [PubMed] [Google Scholar]
- Price V. L., Gallant J. A. The glucose effect in Bacillus subtilis. Eur J Biochem. 1983 Jul 15;134(1):105–107. doi: 10.1111/j.1432-1033.1983.tb07537.x. [DOI] [PubMed] [Google Scholar]
- Sekiguchi J., Takada N., Okada H. Genes affecting the productivity of alpha-amylase in Bacillus subtilis Marburg. J Bacteriol. 1975 Feb;121(2):688–694. doi: 10.1128/jb.121.2.688-694.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shamanna D. K., Sanderson K. E. Genetics and regulation of D-xylose utilization in Salmonella typhimurium LT2. J Bacteriol. 1979 Jul;139(1):71–79. doi: 10.1128/jb.139.1.71-79.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimotsu H., Henner D. J. Construction of a single-copy integration vector and its use in analysis of regulation of the trp operon of Bacillus subtilis. Gene. 1986;43(1-2):85–94. doi: 10.1016/0378-1119(86)90011-9. [DOI] [PubMed] [Google Scholar]
- Shimotsu H., Kuroda M. I., Yanofsky C., Henner D. J. Novel form of transcription attenuation regulates expression the Bacillus subtilis tryptophan operon. J Bacteriol. 1986 May;166(2):461–471. doi: 10.1128/jb.166.2.461-471.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Wilhelm M., Hollenberg C. P. Nucleotide sequence of the Bacillus subtilis xylose isomerase gene: extensive homology between the Bacillus and Escherichia coli enzyme. Nucleic Acids Res. 1985 Aug 12;13(15):5717–5722. doi: 10.1093/nar/13.15.5717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelm M., Hollenberg C. P. Selective cloning of Bacillus subtilis xylose isomerase and xylulokinase in Escherichia coli genes by IS5-mediated expression. EMBO J. 1984 Nov;3(11):2555–2560. doi: 10.1002/j.1460-2075.1984.tb02173.x. [DOI] [PMC free article] [PubMed] [Google Scholar]