Abstract
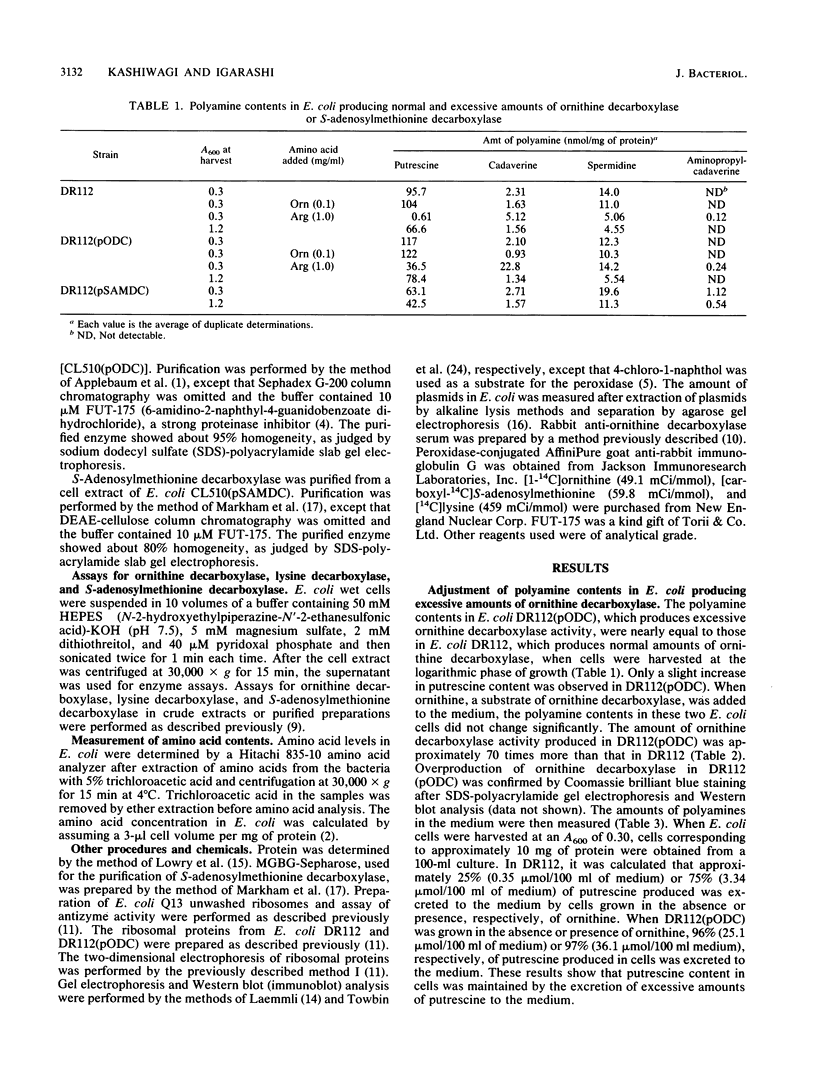
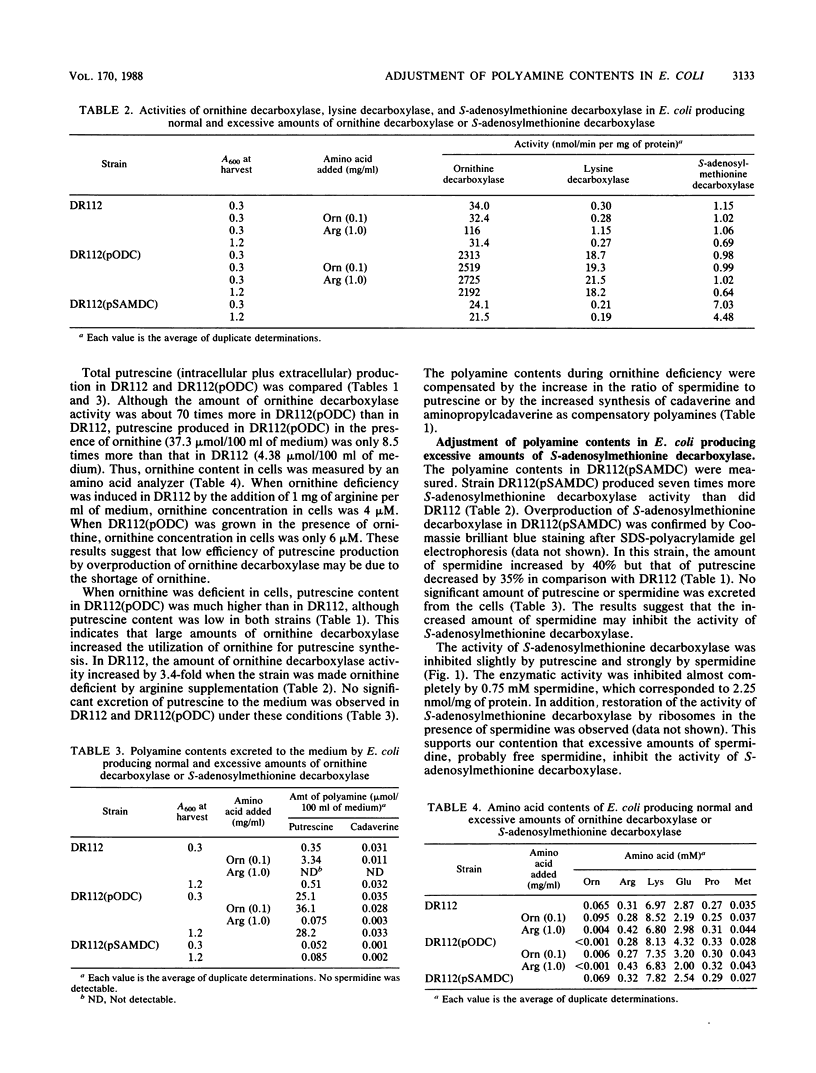
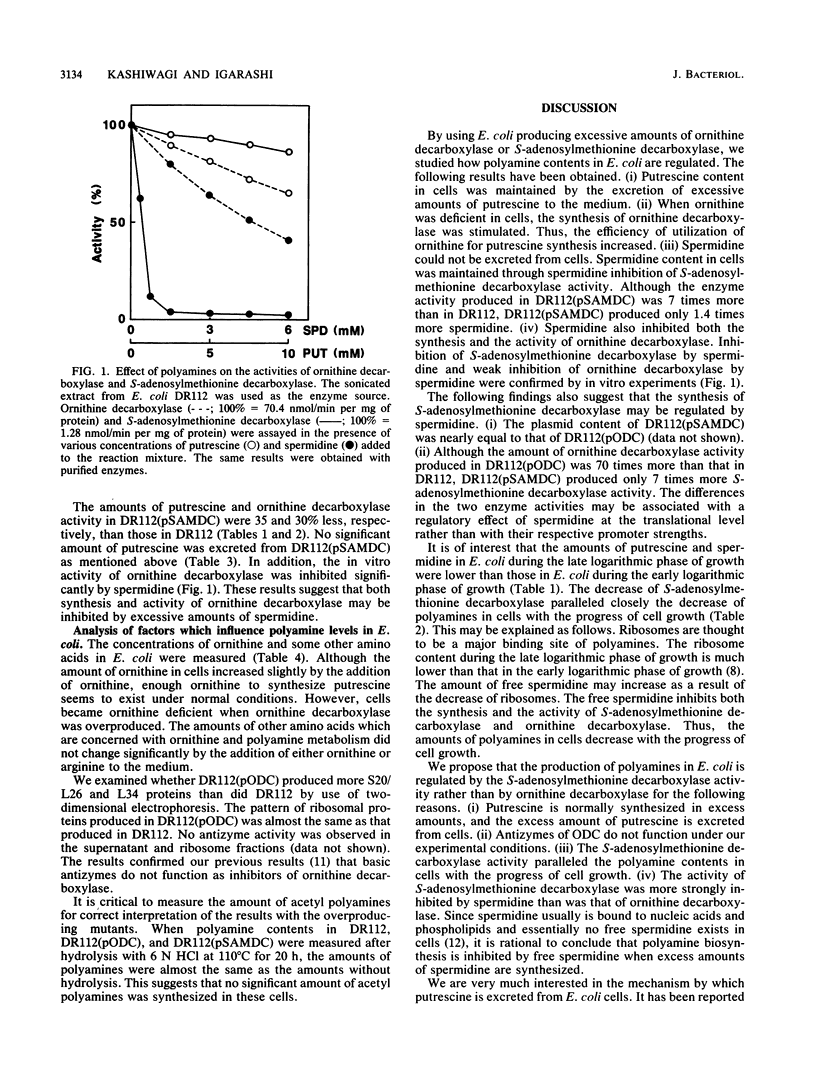
Adjustment of polyamine contents in Escherichia coli was studied with strains of Escherichia coli producing normal (DR112) and excessive amounts of ornithine decarboxylase [DR112(pODC)] or S-adenosylmethionine decarboxylase [DR112(pSAMDC)]. Although DR112(pODC) produced approximately 70 times more ornithine decarboxylase than DR112 did, the amounts of polyamines in the cells of both strains did not change significantly. The amounts of polyamines in DR112(pODC) were adjusted by excretion of excessive amounts of putrescine to the medium. When ornithine was deficient in cells, polyamine contents in DR112(pODC) were much higher than those in DR112, although polyamine contents were low in both strains. This indicates that large amounts of ornithine decarboxylase increased the utilization of ornithine for putrescine synthesis. During ornithine deficiency, strain DR112 produced 3.4 times more ornithine decarboxylase. Strain DR112(pSAMDC) produced seven times more S-adenosylmethionine decarboxylase than DR112 did. In DR112(pSAMDC) an increase (40%) in spermidine content, a decrease (35%) in putrescine content, and no significant excretion of putrescine and spermidine were observed. The amount of ornithine decarboxylase in DR112(pSAMDC) was approximately 30% less than that in DR112. In addition, S-adenosylmethionine decarboxylase activity was strongly inhibited by spermidine. A possible regulatory mechanism to maintain polyamine contents in Escherichia coli is discussed based on the results.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Applebaum D. M., Dunlap J. C., Morris D. R. Comparison of the biosynthetic and biodegradative ornithine decarboxylases of Escherichia coli. Biochemistry. 1977 Apr 19;16(8):1580–1584. doi: 10.1021/bi00627a008. [DOI] [PubMed] [Google Scholar]
- Bakker E. P., Mangerich W. E. Interconversion of components of the bacterial proton motive force by electrogenic potassium transport. J Bacteriol. 1981 Sep;147(3):820–826. doi: 10.1128/jb.147.3.820-826.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle S. M., Markham G. D., Hafner E. W., Wright J. M., Tabor H., Tabor C. W. Expression of the cloned genes encoding the putrescine biosynthetic enzymes and methionine adenosyltransferase of Escherichia coli (speA, speB, speC and metK). Gene. 1984 Oct;30(1-3):129–136. doi: 10.1016/0378-1119(84)90113-6. [DOI] [PubMed] [Google Scholar]
- Fujii S., Hitomi Y. New synthetic inhibitors of C1r, C1 esterase, thrombin, plasmin, kallikrein and trypsin. Biochim Biophys Acta. 1981 Oct 13;661(2):342–345. doi: 10.1016/0005-2744(81)90023-1. [DOI] [PubMed] [Google Scholar]
- Hawkes R., Niday E., Gordon J. A dot-immunobinding assay for monoclonal and other antibodies. Anal Biochem. 1982 Jan 1;119(1):142–147. doi: 10.1016/0003-2697(82)90677-7. [DOI] [PubMed] [Google Scholar]
- Hölttä E., Jänne J., Pispa J. Ornithine decarboxylase from Escherichia coli: stimulation of the enzyme activity by nucleotides. Biochem Biophys Res Commun. 1972 Jun 9;47(5):1165–1171. doi: 10.1016/0006-291x(72)90957-6. [DOI] [PubMed] [Google Scholar]
- Hölttä E., Jänne J., Pispa J. The regulation of polyamine synthesis during the stringent control in Escherichia coli. Biochem Biophys Res Commun. 1974 Aug 5;59(3):1104–1111. doi: 10.1016/s0006-291x(74)80092-6. [DOI] [PubMed] [Google Scholar]
- Igarashi K., Hara K., Watanabe Y., Hirose S., Takeda Y. Polyamine and magnesium contents and polypeptide synthesis as a function of cell growth. Biochem Biophys Res Commun. 1975 Jan 2;64(3):897–904. doi: 10.1016/0006-291x(75)90132-1. [DOI] [PubMed] [Google Scholar]
- Igarashi K., Kashiwagi K., Hamasaki H., Miura A., Kakegawa T., Hirose S., Matsuzaki S. Formation of a compensatory polyamine by Escherichia coli polyamine-requiring mutants during growth in the absence of polyamines. J Bacteriol. 1986 Apr;166(1):128–134. doi: 10.1128/jb.166.1.128-134.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igarashi K., Terada K., Tango Y., Katakura K., Hirose S. Demonstration by affinity chromatography of the cell-free synthesis of ribonuclease-specific immunoglobulin. J Biochem. 1975 Feb;77(2):383–390. doi: 10.1093/oxfordjournals.jbchem.a130736. [DOI] [PubMed] [Google Scholar]
- Kashiwagi K., Igarashi K. Nonspecific inhibition of Escherichia coli ornithine decarboxylase by various ribosomal proteins: detection of a new ribosomal protein possessing strong antizyme activity. Biochim Biophys Acta. 1987 Jan 30;911(2):180–190. doi: 10.1016/0167-4838(87)90007-0. [DOI] [PubMed] [Google Scholar]
- Kashiwagi K., Kobayashi H., Igarashi K. Apparently unidirectional polyamine transport by proton motive force in polyamine-deficient Escherichia coli. J Bacteriol. 1986 Mar;165(3):972–977. doi: 10.1128/jb.165.3.972-977.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyriakidis D. A., Heller J. S., Canellakis E. S. Modulation of ornithine decarboxylase activity in Escherichia coli by positive and negative effectors. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4699–4703. doi: 10.1073/pnas.75.10.4699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Markham G. D., Tabor C. W., Tabor H. S-adenosylmethionine decarboxylase (Escherichia coli). Methods Enzymol. 1983;94:228–230. doi: 10.1016/s0076-6879(83)94039-9. [DOI] [PubMed] [Google Scholar]
- Panagiotidis C. A., Canellakis E. S. Comparison of the basic Escherichia coli antizyme 1 and antizyme 2 with the ribosomal proteins S20/L26 and L34. J Biol Chem. 1984 Dec 25;259(24):15025–15027. [PubMed] [Google Scholar]
- Sakai T. T., Cohen S. S. Regulation of ornithine decarboxylase activity by guanine nucleotides: in vivo test in potassium-depleted Escherichia coli. Proc Natl Acad Sci U S A. 1976 Oct;73(10):3502–3505. doi: 10.1073/pnas.73.10.3502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabor C. W., Tabor H., Hafner E. W., Markham G. D., Boyle S. M. Cloning of the Escherichia coli genes for the biosynthetic enzymes for polyamines. Methods Enzymol. 1983;94:117–121. doi: 10.1016/s0076-6879(83)94019-3. [DOI] [PubMed] [Google Scholar]
- Tabor C. W., Tabor H. Polyamines in microorganisms. Microbiol Rev. 1985 Mar;49(1):81–99. doi: 10.1128/mr.49.1.81-99.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabor C. W., Tabor H. Polyamines. Annu Rev Biochem. 1984;53:749–790. doi: 10.1146/annurev.bi.53.070184.003533. [DOI] [PubMed] [Google Scholar]
- Tabor H., Tabor C. W. Formation of 1,4-diaminobutane and of spermidine by an ornithine auxotroph of Escherichia coli grown on limiting ornithine or arginine. J Biol Chem. 1969 May 10;244(9):2286–2292. [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]


