Abstract
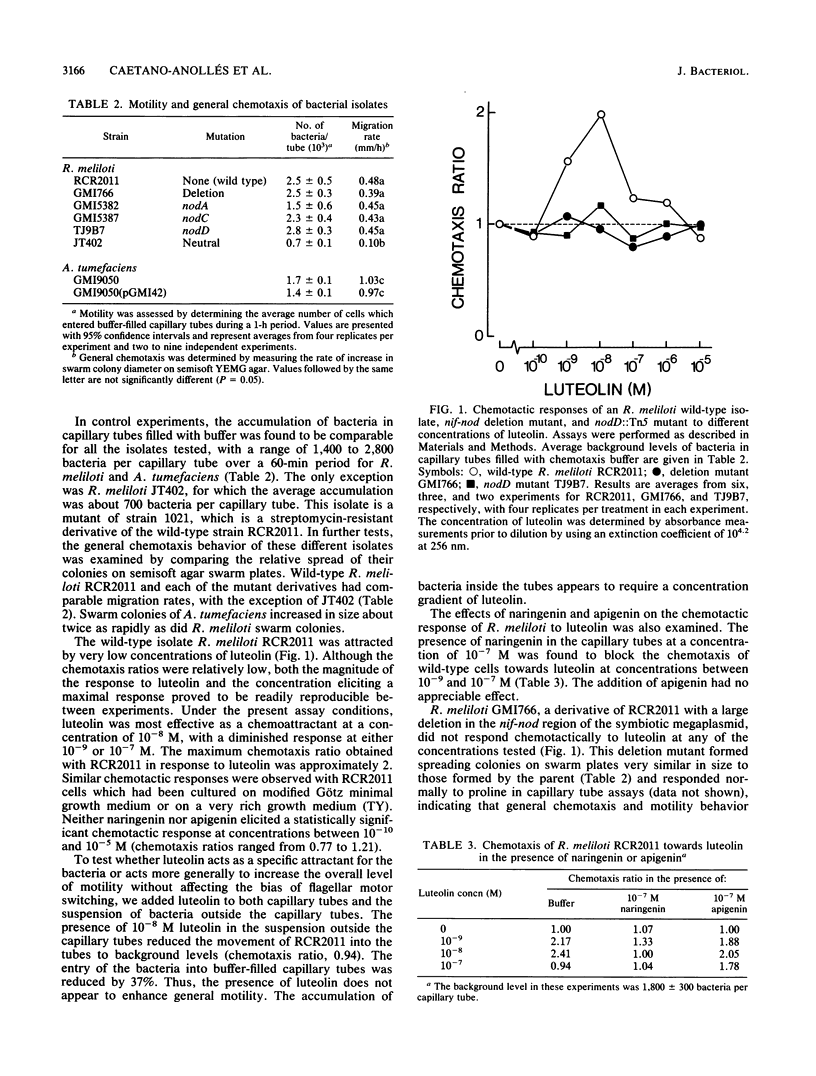
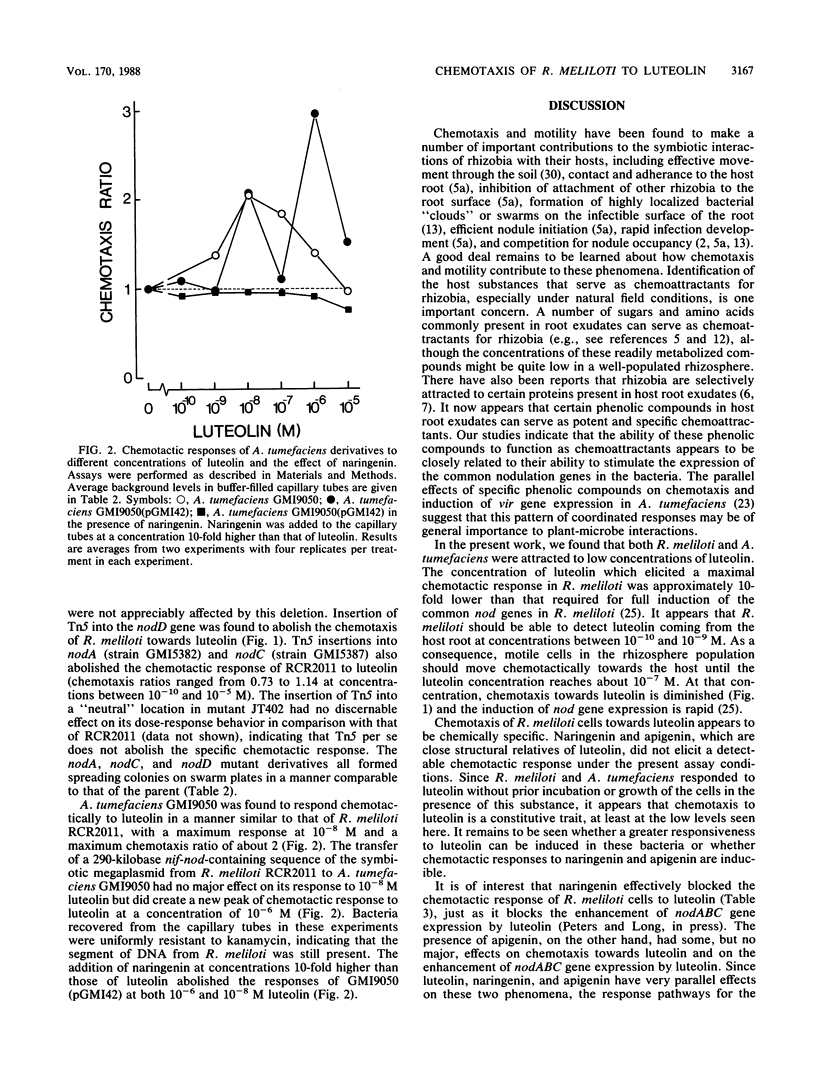
Luteolin is a phenolic compound from plants that acts as a potent and specific inducer of nodABC gene expression in Rhizobium meliloti. We have found that R. meliloti RCR2011 exhibits positive chemotaxis towards luteolin. A maximum chemotactic response was observed at 10(-8) M. Two closely related flavonoids, naringenin and apigenin, were not chemoattractants. The presence of naringenin but not apigenin abolished chemotaxis of R. meliloti towards luteolin. A large deletion in the nif-nod region of the symbiotic megaplasmid eliminated all chemotactic response to luteolin but did not affect general chemotaxis, as indicated by swarm size on semisoft agar plates and chemotaxis towards proline in capillary tubes. Transposon Tn5 mutations in nodD, nodA, or nodC selectively abolished the chemotactic response of R. meliloti to luteolin. Agrobacterium tumefaciens GMI9050, a derivative of the C58 wild type lacking a Ti plasmid, responded chemotactically to 10(-8) M luteolin. The introduction of a 290-kilobase nif-nod-containing sequence of DNA from R. meliloti into A. tumefaciens GMI9050 enabled the recipient to respond to luteolin at concentrations peaking at 10(-6) M as well as at concentrations peaking at 10(-8) M. The response of A. tumefaciens GMI9050 to luteolin was also abolished by the presence of naringenin.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler J. A method for measuring chemotaxis and use of the method to determine optimum conditions for chemotaxis by Escherichia coli. J Gen Microbiol. 1973 Jan;74(1):77–91. doi: 10.1099/00221287-74-1-77. [DOI] [PubMed] [Google Scholar]
- Ames P., Bergman K. Competitive advantage provided by bacterial motility in the formation of nodules by Rhizobium meliloti. J Bacteriol. 1981 Nov;148(2):728–p. doi: 10.1128/jb.148.2.728-729.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergman K., Gulash-Hoffee M., Hovestadt R. E., Larosiliere R. C., Ronco P. G., 2nd, Su L. Physiology of behavioral mutants of Rhizobium meliloti: evidence for a dual chemotaxis pathway. J Bacteriol. 1988 Jul;170(7):3249–3254. doi: 10.1128/jb.170.7.3249-3254.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caetano-Anollés G., Wall L. G., De Micheli A. T., Macchi E. M., Bauer W. D., Favelukes G. Role of Motility and Chemotaxis in Efficiency of Nodulation by Rhizobium meliloti. Plant Physiol. 1988 Apr;86(4):1228–1235. doi: 10.1104/pp.86.4.1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Currier W. W., Strobel G. A. Chemotaxis of Rhizobium spp. to Plant Root Exudates. Plant Physiol. 1976 May;57(5):820–823. doi: 10.1104/pp.57.5.820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Currier W. W., Strobel G. A. Chemotaxis of Rhizobium spp. to a Glycoprotein Produced by Birdsfoot Trefoil Roots. Science. 1977 Apr 22;196(4288):434–436. doi: 10.1126/science.196.4288.434. [DOI] [PubMed] [Google Scholar]
- Debellé F., Rosenberg C., Vasse J., Maillet F., Martinez E., Dénarié J., Truchet G. Assignment of symbiotic developmental phenotypes to common and specific nodulation (nod) genetic loci of Rhizobium meliloti. J Bacteriol. 1986 Dec;168(3):1075–1086. doi: 10.1128/jb.168.3.1075-1086.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djordjevic M. A., Redmond J. W., Batley M., Rolfe B. G. Clovers secrete specific phenolic compounds which either stimulate or repress nod gene expression in Rhizobium trifolii. EMBO J. 1987 May;6(5):1173–1179. doi: 10.1002/j.1460-2075.1987.tb02351.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulash M., Ames P., Larosiliere R. C., Bergman K. Rhizobia are attracted to localized sites on legume roots. Appl Environ Microbiol. 1984 Jul;48(1):149–152. doi: 10.1128/aem.48.1.149-152.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath B., Bachem C. W., Schell J., Kondorosi A. Host-specific regulation of nodulation genes in Rhizobium is mediated by a plant-signal, interacting with the nodD gene product. EMBO J. 1987 Apr;6(4):841–848. doi: 10.1002/j.1460-2075.1987.tb04829.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs T. W., Egelhoff T. T., Long S. R. Physical and genetic map of a Rhizobium meliloti nodulation gene region and nucleotide sequence of nodC. J Bacteriol. 1985 May;162(2):469–476. doi: 10.1128/jb.162.2.469-476.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosslak R. M., Bookland R., Barkei J., Paaren H. E., Appelbaum E. R. Induction of Bradyrhizobium japonicum common nod genes by isoflavones isolated from Glycine max. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7428–7432. doi: 10.1073/pnas.84.21.7428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulligan J. T., Long S. R. Induction of Rhizobium meliloti nodC expression by plant exudate requires nodD. Proc Natl Acad Sci U S A. 1985 Oct;82(19):6609–6613. doi: 10.1073/pnas.82.19.6609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palleroni N. J. Chamber for bacterial chemotaxis experiments. Appl Environ Microbiol. 1976 Nov;32(5):729–730. doi: 10.1128/aem.32.5.729-730.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parke D., Ornston L. N., Nester E. W. Chemotaxis to plant phenolic inducers of virulence genes is constitutively expressed in the absence of the Ti plasmid in Agrobacterium tumefaciens. J Bacteriol. 1987 Nov;169(11):5336–5338. doi: 10.1128/jb.169.11.5336-5338.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parke D., Rivelli M., Ornston L. N. Chemotaxis to aromatic and hydroaromatic acids: comparison of Bradyrhizobium japonicum and Rhizobium trifolii. J Bacteriol. 1985 Aug;163(2):417–422. doi: 10.1128/jb.163.2.417-422.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters N. K., Frost J. W., Long S. R. A plant flavone, luteolin, induces expression of Rhizobium meliloti nodulation genes. Science. 1986 Aug 29;233(4767):977–980. doi: 10.1126/science.3738520. [DOI] [PubMed] [Google Scholar]
- Rosenberg C., Boistard P., Dénarié J., Casse-Delbart F. Genes controlling early and late functions in symbiosis are located on a megaplasmid in Rhizobium meliloti. Mol Gen Genet. 1981;184(2):326–333. doi: 10.1007/BF00272926. [DOI] [PubMed] [Google Scholar]
- Rossen L., Johnston A. W., Downie J. A. DNA sequence of the Rhizobium leguminosarum nodulation genes nodAB and C required for root hair curling. Nucleic Acids Res. 1984 Dec 21;12(24):9497–9508. doi: 10.1093/nar/12.24.9497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossen L., Shearman C. A., Johnston A. W., Downie J. A. The nodD gene of Rhizobium leguminosarum is autoregulatory and in the presence of plant exudate induces the nodA,B,C genes. EMBO J. 1985 Dec 16;4(13A):3369–3373. doi: 10.1002/j.1460-2075.1985.tb04092.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soby S., Bergman K. Motility and Chemotaxis of Rhizobium meliloti in Soil. Appl Environ Microbiol. 1983 Nov;46(5):995–998. doi: 10.1128/aem.46.5.995-998.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truchet G., Debellé F., Vasse J., Terzaghi B., Garnerone A. M., Rosenberg C., Batut J., Maillet F., Dénarié J. Identification of a Rhizobium meliloti pSym2011 region controlling the host specificity of root hair curling and nodulation. J Bacteriol. 1985 Dec;164(3):1200–1210. doi: 10.1128/jb.164.3.1200-1210.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truchet G., Rosenberg C., Vasse J., Julliot J. S., Camut S., Denarie J. Transfer of Rhizobium meliloti pSym genes into Agrobacterium tumefaciens: host-specific nodulation by atypical infection. J Bacteriol. 1984 Jan;157(1):134–142. doi: 10.1128/jb.157.1.134-142.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaat S. A., Wijffelman C. A., Spaink H. P., van Brussel A. A., Okker R. J., Lugtenberg B. J. Induction of the nodA promoter of Rhizobium leguminosarum Sym plasmid pRL1JI by plant flavanones and flavones. J Bacteriol. 1987 Jan;169(1):198–204. doi: 10.1128/jb.169.1.198-204.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler R. J., Peirce C., Bergman K. Mapping and cloning of a fla-che region of the Rhizobium meliloti chromosome. J Bacteriol. 1986 Nov;168(2):785–790. doi: 10.1128/jb.168.2.785-790.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]


