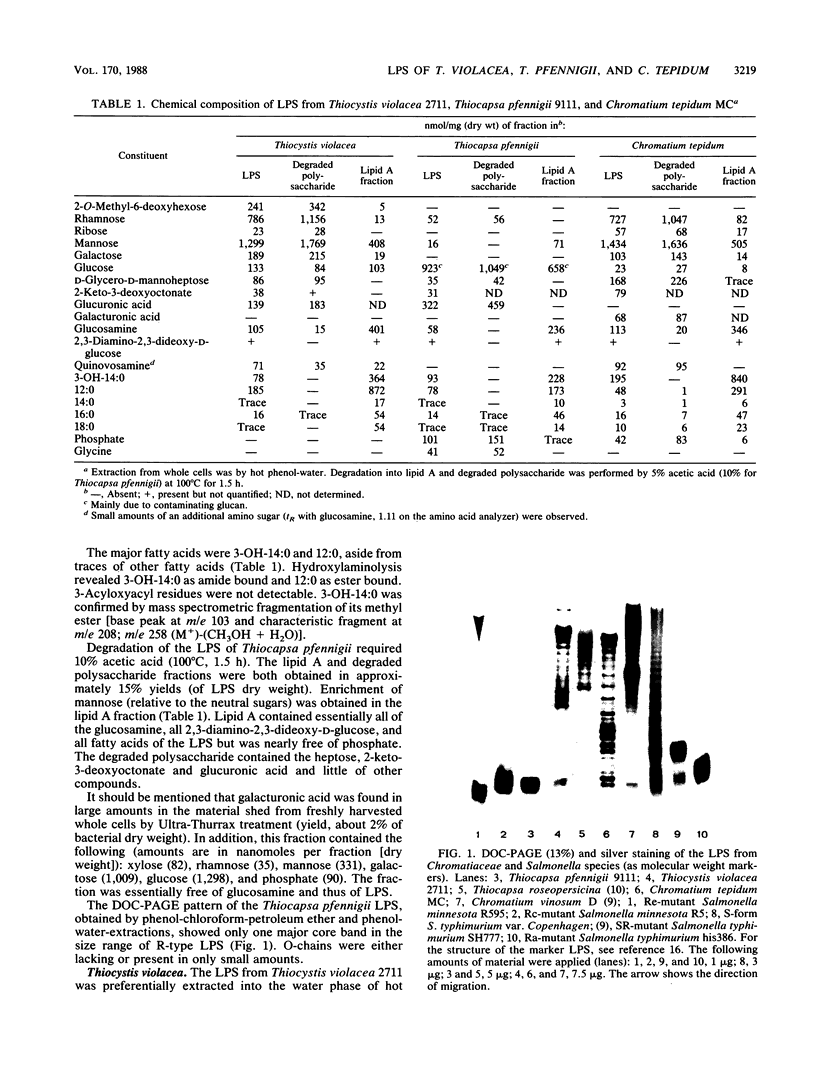
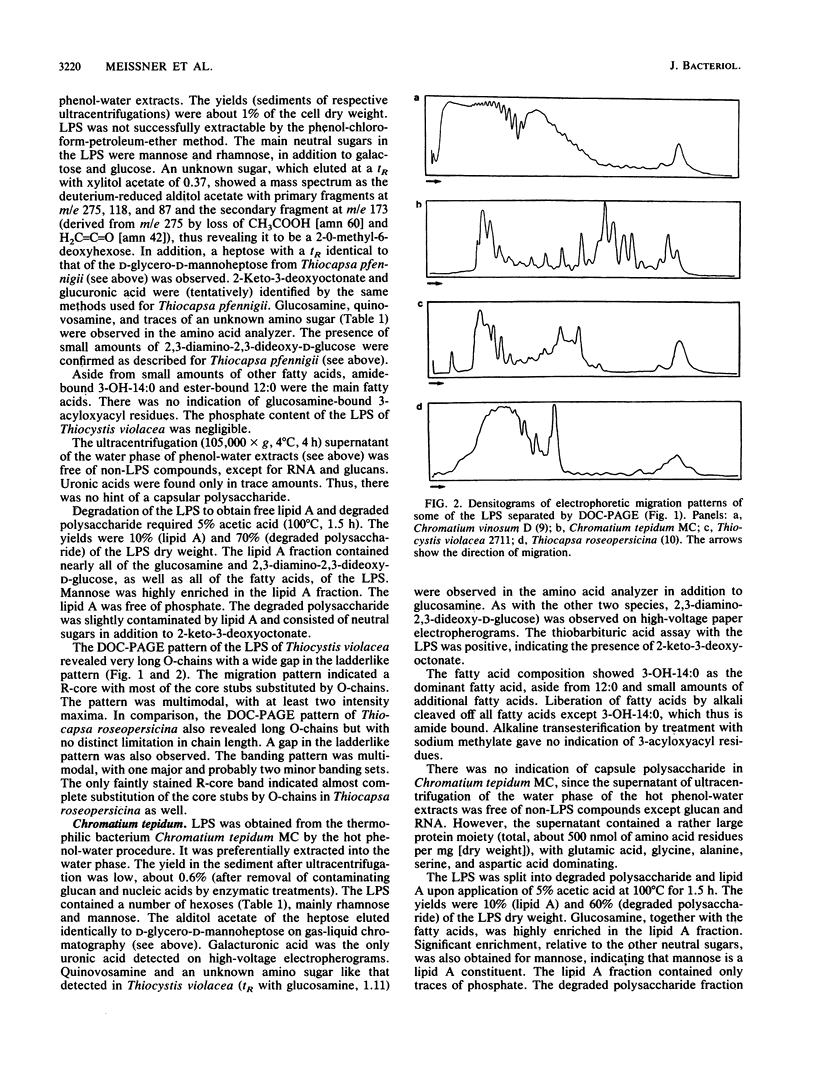
Abstract
The lipopolysaccharides (LPS) of three species of purple sulfur bacteria (Chromatiaceae), Thiocystis violacea, Thiocapsa pfennigii, and the moderately thermophilic bacterium Chromatium tepidum, were isolated. The LPS of Thiocystis violacea and Chromatium tepidum contained typical O-specific sugars, indicating O-chains. Long O-chains were confirmed for these species by sodium deoxycholate gel electrophoresis of their LPS. Thiocapsa pfennigii, however, had short or no O-chains. The core region of the LPS of all three species comprised D-glycero-D-mannoheptose as the only heptose and 2-keto-3-deoxyoctonate. The lipid A, obtained from the LPS by mild acid hydrolysis, contained glucosamine as the main amino sugar. Amide-bound 3-hydroxymyristic acid was the only hydroxy fatty acid. The main ester-bound fatty acid in all lipid A fractions was 12:0. Mannose and small amounts of 2,3-diamino-2,3-dideoxy-D-glucose were common constituents of the lipid A of the three Chromatiaceae species investigated. All lipid A fractions were essentially free of phosphate.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blumenkrantz N., Asboe-Hansen G. New method for quantitative determination of uronic acids. Anal Biochem. 1973 Aug;54(2):484–489. doi: 10.1016/0003-2697(73)90377-1. [DOI] [PubMed] [Google Scholar]
- Brade H., Galanos C. A method to detect 2-keto-3-deoxyoctanat and related compounds on pherograms and chromatograms. Anal Biochem. 1983 Jul 1;132(1):158–159. doi: 10.1016/0003-2697(83)90440-2. [DOI] [PubMed] [Google Scholar]
- Fox G. E., Stackebrandt E., Hespell R. B., Gibson J., Maniloff J., Dyer T. A., Wolfe R. S., Balch W. E., Tanner R. S., Magrum L. J. The phylogeny of prokaryotes. Science. 1980 Jul 25;209(4455):457–463. doi: 10.1126/science.6771870. [DOI] [PubMed] [Google Scholar]
- Galanos C., Lüderitz O., Westphal O. A new method for the extraction of R lipopolysaccharides. Eur J Biochem. 1969 Jun;9(2):245–249. doi: 10.1111/j.1432-1033.1969.tb00601.x. [DOI] [PubMed] [Google Scholar]
- Hurlbert R. E., Hurlbert I. M. Biological and physicochemical properties of the lipopolysaccharide of Chromatium vinosum. Infect Immun. 1977 Jun;16(3):983–994. doi: 10.1128/iai.16.3.983-994.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurlbert R. E., Weckesser J., Mayer H., Fromme I. Isolation and characterization of the lipopolysaccharide of Chromatium vinosum. Eur J Biochem. 1976 Sep 15;68(2):365–371. doi: 10.1111/j.1432-1033.1976.tb10823.x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROBERTS N. R., LEINER K. Y., WU M. L., FARR A. L. The quantitative histochemistry of brain. I. Chemical methods. J Biol Chem. 1954 Mar;207(1):1–17. [PubMed] [Google Scholar]
- Niedermeier W., Tomana M. Gas chromatographic analysis of hexosamines in glycoproteins. Anal Biochem. 1974 Feb;57(2):363–368. doi: 10.1016/0003-2697(74)90090-6. [DOI] [PubMed] [Google Scholar]
- Roppel J., Mayer H. Identification of a 2, 3-diamino-2, 3-dideoxyhexose in the lipid A component of lipopolysaccharides of Rhodopseudomonas viridis and Rhodopseudomonas palustris. Carbohydr Res. 1975 Mar;40(1):31–40. doi: 10.1016/s0008-6215(00)82666-x. [DOI] [PubMed] [Google Scholar]
- SNYDER F., STEPHENS N. A simplified spectrophotometric determination of ester groups in lipids. Biochim Biophys Acta. 1959 Jul;34:244–245. doi: 10.1016/0006-3002(59)90255-0. [DOI] [PubMed] [Google Scholar]
- Sleytr U. B., Messner P. Crystalline surface layers on bacteria. Annu Rev Microbiol. 1983;37:311–339. doi: 10.1146/annurev.mi.37.100183.001523. [DOI] [PubMed] [Google Scholar]
- TREVELYAN W. E., PROCTER D. P., HARRISON J. S. Detection of sugars on paper chromatograms. Nature. 1950 Sep 9;166(4219):444–445. doi: 10.1038/166444b0. [DOI] [PubMed] [Google Scholar]
- Takacs B. J., Holt S. C. Thiocapsa floridana; a cytological, physical and chemical characterization. I. Cytology of whole cells and isolated chromatophore membranes. Biochim Biophys Acta. 1971 Apr 13;233(2):258–277. doi: 10.1016/0005-2736(71)90325-7. [DOI] [PubMed] [Google Scholar]
- Tsai C. M., Frasch C. E. A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal Biochem. 1982 Jan 1;119(1):115–119. doi: 10.1016/0003-2697(82)90673-x. [DOI] [PubMed] [Google Scholar]
- WARAVDEKAR V. S., SASLAW L. D. A sensitive colorimetric method for the estimation of 2-deoxy sugars with the use of the malonaldehyde-thiobarbituric acid reaction. J Biol Chem. 1959 Aug;234(8):1945–1950. [PubMed] [Google Scholar]
- Weckesser J., Drews G., Mayer H. Lipopolysaccharides of photosynthetic prokaryotes. Annu Rev Microbiol. 1979;33:215–239. doi: 10.1146/annurev.mi.33.100179.001243. [DOI] [PubMed] [Google Scholar]
- Woese C. R., Stackebrandt E., Macke T. J., Fox G. E. A phylogenetic definition of the major eubacterial taxa. Syst Appl Microbiol. 1985;6:143–151. doi: 10.1016/s0723-2020(85)80047-3. [DOI] [PubMed] [Google Scholar]
- Woese C. R., Stackebrandt E., Weisburg W. G., Paster B. J., Madigan M. T., Fowler V. J., Hahn C. M., Blanz P., Gupta R., Nealson K. H. The phylogeny of purple bacteria: the alpha subdivision. Syst Appl Microbiol. 1984;5:315–326. doi: 10.1016/s0723-2020(84)80034-x. [DOI] [PubMed] [Google Scholar]
- Wollenweber H. W., Broady K. W., Lüderitz O., Rietschel E. T. The chemical structure of lipid A. Demonstration of amide-linked 3-acyloxyacyl residues in Salmonella minnesota Re lipopolysaccharide. Eur J Biochem. 1982 May;124(1):191–198. doi: 10.1111/j.1432-1033.1982.tb05924.x. [DOI] [PubMed] [Google Scholar]