Abstract
Syphilis, a sexually transmitted infection caused by the spirochetal bacterium Treponema pallidum, remains a global public health problem. T. pallidum is believed to be an extracellular pathogen and, as such, the identification of T. pallidum outer membrane proteins that could serve as targets for opsonic or bactericidal antibodies has remained a high research priority for vaccine development. However, the identification of T. pallidum outer membrane proteins has remained highly elusive. Recent studies and bioinformatics have implicated four treponemal proteins as potential outer membrane proteins (TP0155, TP0326, TP0483 and TP0956). Indirect immunofluorescence assays performed on treponemes encapsulated within agarose gel microdroplets failed to provide evidence that any of these four molecules were surface-exposed in T. pallidum. Second, recombinant fusion proteins corresponding to all four candidate outer membrane proteins were used separately, or in combination, to vaccinate New Zealand White rabbits. Despite achieving high titers (>1:50,000) of serum antibodies, none of the rabbits displayed chancre immunity after intradermal challenge with viable T. pallidum.
Keywords: TREPONEMA PALLIDUM, VACCINE, OUTER MEMBRANE PROTEIN
1. Introduction
Syphilis is a chronic, complex sexually transmitted infection of humans caused by the spirochetal bacterium Treponema pallidum subspecies pallidum (Tp). Although effective drug-treatment regimes exist, syphilis remains a pandemic disease [1] that contributes to the spread of HIV [2]. The development of an efficacious vaccine has remained an important research and public health goal [3-6].
Protective immunity against Tp infection is supported by clinical observations in both humans and animals. Although derived from a study in which ethics were compromised, humans with late latent syphilis appear to develop some immunity to subsequent infection [7]. Regarding animal models, rabbits are the preferred mammalian model for immunoprotection studies with Tp because they develop chancre-like lesions after intradermal injection [3, 8]. Chancre immunity is achievable in rabbits systemically infected with Tp for 3-6 months and is characterized by the failure to produce lesions after intradermal challenge with virulent Tp [3, 9]. Furthermore, a seminal study by J.N. Miller [4] showed that rabbits immunized over 37 weeks with multiple injections of gamma-irradiated (killed) Tp developed full immunity to subsequent challenge with viable Tp, thereby substantiating the feasibility of a syphilis vaccine.
Considering that Tp is believed to be an extracellular pathogen, it is plausible that antibodies play a pivotal role in the clearance of the spirochetes. The primary targets of these antibodies are thought to be surface-exposed, outer membrane proteins (OMPs). However, the identification of bona fide OMPs in Tp has remained elusive [5, 10-12]. Towards that goal, four Tp proteins, TP0155, TP0326, TP0483, and TP0956, have recently emerged as potential OMP candidates. TP0155 and TP0483 were reported as fibronectin-binding proteins [13]. Antibodies against TP0326 were opsonic and enhanced the phagocytocis of Tp by macrophages [5]. Finally, rising antibody titers against TP0956 in rabbits infected with Tp correlated with the development of chancre immunity, implying a possible contribution of these antibodies to immune protection [14]. Unfortunately, despite these promising aforementioned results, the membrane topologies of these four proteins remain unknown, as well as their potential to serve as highly efficacious vaccinogens for the prevention of syphilitic infection. To further examine the potential cell-surface exposure of TP0155, TP0326, TP0483, and TP0956, antibodies directed against each respective protein were employed in the agarose gel microdroplet immunofluorescence assay [15]. We also evaluated each protein individually, or combined as a quadravalent vaccine, for immunoprotective capability in the rabbit model of experimental syphilis.
2. Materials and Methods
2.1. Cultivation and isolation of treponemes
All animal studies described herein were approved by the U.T. Southwestern Institutional Animal Care and Use Committee.
Tp subspecies pallidum (Nichols strain) was maintained and passaged by intratesticular inoculation of adult male New Zealand White rabbits as previously described [15]. Rabbit testicular debris was removed from treponemal suspensions by two successive rounds of slow-speed centrifugation (200 × g for 8 minutes).
2.2. Generation of histidine-tagged fusion proteins for vaccination studies
Individual open-reading frames lacking N-terminal signal sequences were amplified by PCR from Tp genomic DNA using the following primer pairs: 5'-TP0155 5'-ACGCGGATCCCCATTGACACCTGCCCTCAC-3' and 3'-TP0155 5'-ATCCAAGCTTTTACTACGGAAGGGTACGCATACG-3'; 5'-TP0483 5'-ACGCGGATCCAAGGAACTCGTCCACGTATCTCAG-3' and 3'-TP0483 5'-ATCCAAGCTTTTATCAGTTATGAAAGCGATAGCCG-3'; 5'-TP0956 5'-ACGCGGATCCCTCTCCCACACGCTCGCTC-3' and 3'-TP0956 5'-ATCCAAGCTTTTATCAATCCAAGAAAAAATCCTGC-3'; 5'-TP0326 5'-ACGCGGATCCGAGGGAAAGCCTATCTCTG-3' and 3'-TP0326 5'-ATCCAAGCTTTTACTACAAATTATTTACCGTGAAC-3'. Restriction sites (BamH I or Hind III) are underlined. N-terminal, histidine-tagged fusion proteins were generated using pProEX-HTb (Invitrogen, Calsbad, CA). Constructs were transformed into Escherichia coli XL1-Blue (Stratagene, La Jolla, CA) and insert sequences verified via DNA sequencing. Recombinant fusion proteins were purified on nickel-NTA agarose (Qiagen, Valencia, CA) following manufacturer's directions. Further purification was achieved by electroeluction of SDS-PAGE-separated protein into SDS-PAGE running buffer (192 mM glycine, 25 mM Tris base, 0.1% SDS) using an Elutrap electroelution system (Schleicher and Schuell Inc., Keene, NH).
2.3. SDS-PAGE and immunoblotting
Proteins were separated on 12.5% polyacrylamide resolving gels and were transferred electrophoretically to a nitrocellulose membrane (Schleicher & Schuell) for immunoblotting. Blots were blocked for 1 h in StartingBlock (Pierce, Rockford, IL), incubated for 1 h with a 1:1000-1:50,000 dilution of rabbit polyclonal antiserum or a 1:1000 dilution of rat polyclonal antiserum or mouse monoclonal, 11E3 [16]. After 3 washes in phosphate-buffered saline (PBS)/0.5%Tween, blots were incubated for 1 h with 1:1000-1:20,000 dilution of horseradish peroxidase conjugated goat anti-rabbit (rat or mouse as appropriate) immunoglobulin G (IgG) (Jackson ImmunoResearch, West Grove, PA). Immunoblots were either developed with 4-chloro-1-naphthol as the substrate, or with the ECL Western Blotting Detection Kit (Amersham Biosciences, Piscataway, NJ) for chemiluminescence detection.
2.4. Rat antisera generation
For the generation of polyclonal antisera, 20 μg of each recombinant protein (in 200 μl of PBS) was emulsified with an equal volume of complete Freund's adjuvant (Sigma, St. Louis, MO) and injected intraperitoneally into 6-week-old, female Sprague-Dawley rats (Harlan, Indianapolis, IN). After 3 weeks, the rats were boosted with a similar amount of protein emulsified in incomplete Freund's adjuvant (Sigma). Two weeks after this boost, antiserum was collected and immunoblotted against recombinant protein and, if necessary, a second boost was performed to increase antiserum affinity and titer. Serum antibody titers were estimated by preparing 10-fold serial dilutions of each antiserum, and using them in colorimetric immunoblots against 500 ng (per gel lane) of each protein. Antibody titers were designated as greater than the lowest serum dilution that yielded a visible (positive) band on immunoblots.
2.5. Rabbit immunization and intradermal challenge
Each protein antigen was emulsified in a 1:1 mixture of the Ribi adjuvant system (Sigma). New Zealand White male rabbits were injected with 125 μg of test antigen at each immunization, divided equally among 6 intradermal, 1 subcutaneous, and 2 intramuscular injection sites. Each rabbit was immunized a total of 4 times at three-week intervals. Two rabbits were immunized with one of the following antigens Tp47, TP0155, TP0326, TP0483, or TP0956, and two rabbits were immunized with a mixture of TP0155, TP0326, TP0483, and TP0956. Finally, two rabbits were left unimmunized (negative controls). Antiserum was collected two weeks after the final immunization and titers were evaluated by immunoblot analysis using the relevant immunizing recombinant protein or protein mixture. Rabbits were challenged with 105 freshly-isolated Tp administered intradermally at 8 sites along shaved backs. Rabbits were examined daily and photographed to document lesion development. Darkfield microscopic examinations of lesion aspirates were preformed to confirm the presence of motile spirochetes.
2.6. Encapsulation of Tp within agarose gel microdroplets and indirect immunofluorescence of treponemal antigens
Spirochetes were harvested in Tp medium and flushed with 5% CO2 / 95% N2 [15]. The Tp medium consisted of a combination of Earle's balanced salt solution, MEM amino acids, MEM non-essential amino acids, and MEM vitamins with 2 mM L-glutamine, 550 μM D-mannitol, 320 μM L-histidine, 24 mM sodium bicarbonate, 25 mM MOPS (3-[N-Morpholino]propanesulfonic acid), 900 μM sodium pyruvate , 14 mM D-(+)glucose, 650 μM dithiothreitol, and 20% fetal bovine serum (Mediatech, Inc., Herndon, VA) with a final pH of 7.5. Spirochetes in suspension were enumerated by darkfield microscopy and diluted to a concentration of 108/ml in Tp medium before being incorporated into 2% low-melting temperature agarose as previously described [15].
The agarose gel microdroplet preparation was separated into one ml aliquots for labeling with Tp-specific antibodies. The primary antibodies utilized were: 11E3, a mouse monoclonal antibody directed against Tp47 [16] (1:20 dilution), polyclonal rabbit anti-TP0326 (1:3 dilution), or polyclonal rat anti-TP0155, anti-TP0483 and anti-TP0956 (1:100 dilution). Polyclonal rat serum against Borrelia burgdorferi (B. burgdorferi) OspC (1:100) was used as a non-treponemal negative control. In addition, YOYO-1 (Molecular Probes) (1:1000 dilution), a nuclear stain with a similar staining pattern as Tp47, was also used as an additional negative control (data not shown)[15]. The microdroplets were incubated with primary antibody overnight at 4°C, followed by gentle centrifugation (500 x g, 5 min) and five washes with fresh Tp medium. Conjugated secondary antibodies, either 2 μg of goat anti-mouse IgG, or 2 μg of goat anti-rat or 1 μg of goat anti-rabbit Alexa Fluor 488 or 568 (Molecular Probes), were added to the agarose gel microdroplets and incubated for 2 h at 4°C, followed by washing in Tp medium. All secondary antibody probes (without primary antibody) were utilized alone in gel microdroplet preparations to assess non-specific labeling.
Spirochetes encapsulated within gel microdroplets were visualized on glass slides with an Olympus BH2 microscope (darkfield lens, BP545 [red], and BP490 [green] filter [Olympus America Inc, Melville, NY]). Slides were prepared from at least 3 independent microdroplet preparations, and more than 100 bacteria were counted per slide. Images were obtained using a SPOT Pursuit camera (Diagnostic Instruments, Inc., Sterling Heights, MI). The ratio of fluorescent spirochetes to the total number of spirochetes (determined by darkfield microscopy) was calculated.
3. Results
3.1. Assessment of TP0155, TP0326, TP0483 and TP0956 cell-surface exposure in Tp
The agrose gel microdroplet method [15] was utilized to assess whether TP0155, TP0326, TP0483 or TP0956 are exposed on the surface of Tp. Monospecific rat- or rabbit-derived polyclonal antibodies were employed to detect TP0155, TP0326, TP0483, or TP0956. A monoclonal antibody directed against Tp47, a periplasmic lipoprotein of Tp, was used as a marker for subsurface localization [15]. We first characterized the antibodies used in the gel microdroplet assays by probing Tp whole cell lysates to detect native treponemal proteins. Non-rabbit antibodies are particularly advantageous as probes in gel microdroplet assays because they circumvent the need to use anti-rabbit-conjugated secondary antibody (which can react with rabbit antibody that may contaminate Tp preparations). However, in the case of TP0326, only rabbit antibodies were available. Sensitive immunoblotting (chemiluminescence) was used to assess antigen-specific antibodies in the sera of immunized animals after sera were diluted 1:1000. None of the antisera from unimmunized (pre-immune) animals showed evidence in immunoblots of antibodies directed against either the respective recombinant (immunizing) protein or the corresponding native molecule of predicted size in Tp (Fig. 1A-D, left column) (Table 1). When animals were immunized with each recombinant protein, all elicited antibodies that reacted with their corresponding immunizing recombinant antigen (Fig. 1A-D, right column, right-facing arrows). When these same sera were used in immunoblots with Tp, sera from animals immunized with rTP0483, rTP0956, or rTP0326 showed antibody reactivities with native treponemal polypeptides (Fig. 1B,C, left-facing arrows) that corresponded with the predicted molecular masses of each native protein in Tp (Table 1). Sera from rats immunized with TP0155 revealed a polypeptide in Tp (Fig. 1A, left-facing arrow) slightly higher in molecular mass than predicted to be the case for the cognate native molecule in Tp (Table 1); the reason for this difference remains unclear at this time. Of note, all sera (rat or rabbit, pre-immune or immune) blotted against Tp displayed reactivity with a polypeptide of ca. 50-kDa (rabbit) or ca. 54-kDa (rat). The prominent polypeptide in Fig. 1D is most likely rabbit IgG (50-kDa heavy chain) contaminating the Tp suspensions. However, sera from immunized rats (Fig. 1A-C) contained antibodies that reacted with a slightly larger (54-kDa) ubiquitous polypeptide; this reactivity was faint in pre-immune sera and appeared to increase in intensity after immunization. Finally, as expected [16], murine monoclonal antibody 11E3 detected Tp47 prominently within Tp whole cell lysates (not shown).
Fig. 1.

Chemiluminescent immunoblot analysis of sera from animals immunized with recombinant fusion proteins of TP0155, TP0483, TP0956, or TP0326. In the cases of TP0155 (A), TP0483 (B), and TP0956 (C), individual recombinant (r) proteins or whole cell lysates of 1 × 108 Tp (Tp) were probed using pooled non-immune (normal) rat sera or immune rat sera. In the case of TP0326 (D), recombinant protein or the lysate from 1 × 108 Tp were probed using non-immune rabbit sera or immune rabbit sera. The molecular mass standards (in kDa) are shown at the left of each matched set of images. Right-facing arrows delineate recombinant protein, whereas left-facing arrows highlight proteins in Tp whole cell lysates uniquely reacting with each antibody.
Table 1.
Sizes of Tp proteins.
| Recombinant protein name |
Amino acids in recombinant protein |
Calculated molecular mass of recombinant (includes tag) (kDa) 1 |
Estimated molecular mass of native mature protein (kDa) 2 |
|---|---|---|---|
| rTP0155 | 46-371 | 39.0 | 38.1 |
| rTP0326 | 29-837 | 94.3 | 93.0 |
| rTP0483 | 22-374 | 42.8 | 39.6 |
| rTP0956 | 32-323 | 35.9 | 33.8 |
An N-terminal histidine tag and amino acid linker adds an additional 3.2 kDa to each recombinant protein.
This table does not take into account possible acylation of lipoproteins (i.e., TP0326 and TP0956).
When the antibodies against Tp47, TP0155, TP0326 or TP0483 were used to probe intact Tp encapsulated within agarose gel microdroplets, spirochetes were not labeled (Fig. 2A). In contrast, spirochetes treated with Triton X-100 fluoresced when treated with the same antibody preparations (Fig. 2A). Quantitatively, less than 9% of spirochetes fluoresced when exposed to the antibodies, whereas in the presence of Triton X-100, immunolabeling was observed in no less than 86% of the spirochetes (Fig. 2B). Control labeling experiments using antibodies against an unrelated surface protein from B. burgdorferi (OspC) or only secondary antibodies (anti-mouse or rat) had minimal fluorescence (<1%) in the presence of Triton X-100 (data not shown). However, slightly higher labeling (~10%) was observed using the anti-rabbit secondary antibody alone (Fig. 2B), most likely due to the presence of rabbit host antibodies in the treponemal preparation (Fig. 1D). Interestingly, whereas treponemes did not substantially fluoresce in the absence of Triton X-100 when probed for TP0956 (4% labeling), the addition of detergent did not increase the amount of fluorescence to any appreciable degree (16% labeling).
Fig. 2.
Membrane topology assessment of TP0155, TP0326, TP0483 and TP0956 in Tp. (A) Tp bacteria encapsulated in agarose gel microdroplets were exposed to antibodies (Fig. 1) directed against Tp47 (control), TP0155, TP0326, TP0483 or TP0956 in the presence or absence of 0.15% (v/v) Triton X-100 (TX). After secondary antibody treatment, spirochetes were examined by either darkfield (DF) or fluorescence microscopy. (B) Fluorescent treponemes were quantified from at least 3 independent microdroplet preparations and data were plotted. A control experiment utilizing anti-rabbit secondary antibody only was also plotted for comparison (Rab 2o). Error bars represent standard deviations.
3.2. Rabbit immunizations with histidine-tagged fusion proteins
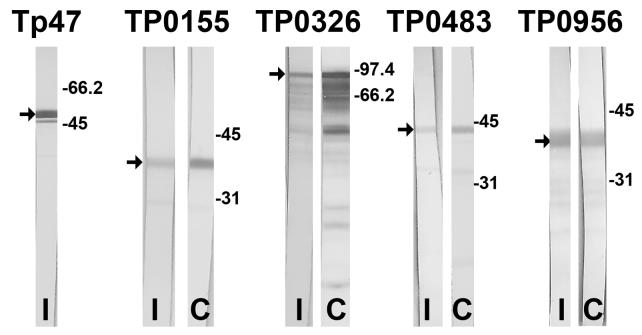
Each of two rabbits was immunized with a total of 500 μg of each antigen, or a combination of TP0155, TP0326, TP0483 and TP0956, over a 9-week period. Assessment of antibody titers within the various immunized rabbit sera was used as a surrogate marker for the immunogenicity of each recombinant antigen preparation. All rabbits immunized with individual proteins or with the tetravalent combination demonstrated serum titers of greater than 1:50,000 for each antigen, as determined by immunoblotting 10-fold serial dilutions of each serum sample. A representative immunoblot from each rabbit pair is shown in Fig. 3. Smaller polypeptides on immunoblots most likely represented fusion protein degradation products.
Fig. 3.
Representative immunoblots (colorimetric) of recombinant Tp proteins using sera from vaccinated rabbits. A 1:50,000 dilution of serum from vaccinated rabbits was used to probe for reactivity with recombinant Tp proteins. An immunoblot using serum from one rabbit immunized with Tp47, TP0155, TP0326, TP0483 or TP0956 (I) is compared to an immunoblot using sera from one rabbit immunized with the combination (C) vaccine consisting of TP0155, TP0326, TP0483 and TP0956. Arrows denote full-length recombinant protein (smaller polypeptides likely represent degradation products). Molecular mass standards (in kDa) are shown at the right of each set of immunoblots.
The rabbit immune responses after receiving the various vaccine preparations were assessed by evaluating antibody reactivities of the pre- and post- immunization sera from each rabbit with recombinant immunogens or Tp whole cell lysates. Sensitive immunoblotting (chemiluminescence) again was used to aid in the detection of potentially low-abundance proteins in Tp. Representative immunoblots for each antigen are shown in Fig. 4. Sera from animals immunized with each respective immunogen showed their expected immunoreactivity with the corresponding recombinant antigen (Fig. 4A-D). Immunoblots using sera from the control immunization of animals with Tp47 yielded the well established pattern of immunoreactivity with Tp47 in Tp (not shown) [16]. Sera from all rabbit immunizations reacted with a protein in Tp preparations migrating at about 50 kDa, which also appeared in immunoblots of sera from non-immune animals, again representing rabbit IgG (heavy chain) within treponemal preparations. Sera from rabbits immunized with TP0326 (Fig. 4B), TP0483 (Fig. 4C), or TP0956 (Fig. 4D) detected native treponemal polypeptides of the expected molecular masses (Table 1), consistent with results of Fig. 1. In contrast, a native polypeptide of expected mass was not detected in Tp when the immunoblots were probed with TP0155-immunized rabbit serum (Fig. 4A). The combined data revealed that vaccination with the respective recombinant proteins gave rise to antibodies reactive with native TP0326, TP0483, and TP0956. Whereas the intrinsic immunogenicity of TP0155 was demonstrated (as per high titers of antibody in the sera of immunized animals) (Figs. 3 and 4A), the failure to identify the cognate polypeptide in Tp likely was due to low abundance [17].
Fig. 4.
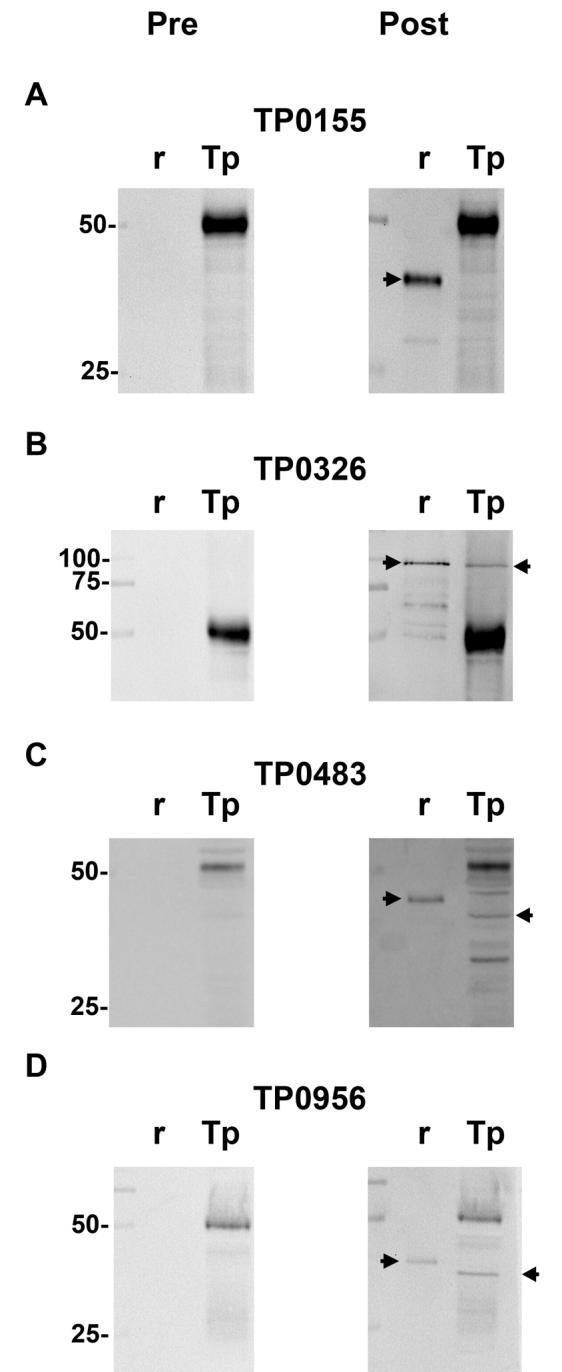
Chemiluminescent immunoblot analysis of Tp whole cell lysates using sera from rabbits vaccinated with recombinant proteins. Individual (r) recombinant proteins or lysates from 1 × 108 Tp (Tp) were probed using rabbit sera harvested before (Pre) or after (Post) vaccination with the recombinant proteins. The molecular mass standards (in kDa) are shown at the left of each matched set of images. Right-facing arrows denote recombinant protein. Left-facing arrows highlight proteins in treponemal whole cell lysates that react uniquely with antibody in the sera of vaccinated rabbits.
The protective capacity of each fusion protein was tested by challenging immunized rabbits with live Tp. All rabbits developed red, raised, indurated lesions at all eight inoculation sites which eventually ulcerated (Fig. 5). Two antigens (TP0326 and TP0956) appeared to cause intensified lesions. Darkfield microscopy performed on aspirates of lesions from all animals revealed the presence of motile spirochetes. Ulcerations on the rabbits inoculated with the control immunogen, Tp47, appeared earlier than in the other immunized rabbits.
Fig. 5.
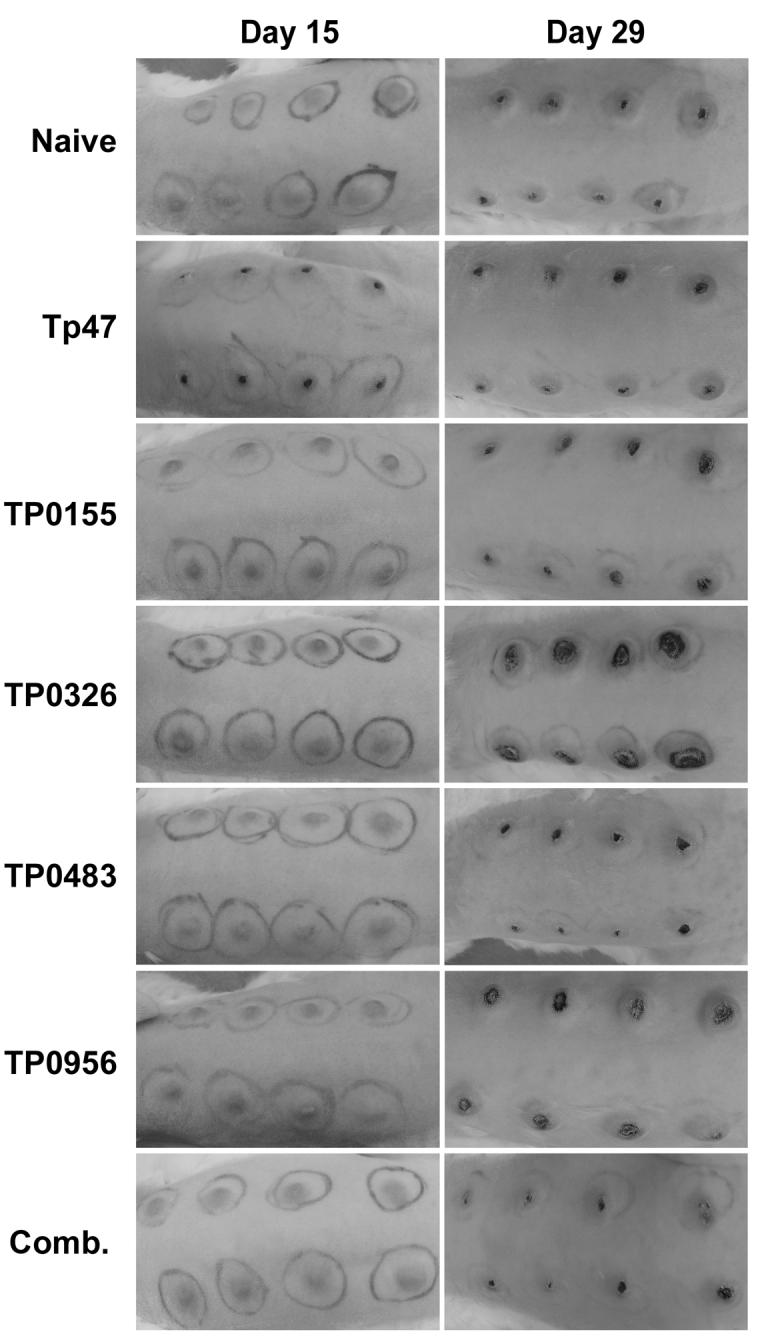
Intradermal challenge of vaccinated rabbits with Tp. Rabbits previously vaccinated with the recombinant Tp protein antigens Tp47, TP0155, TP0326, TP0483, or TP0956, or a combination (Comb.) of TP0155, TP0326, TP0483 and TP0956, were challenged intradermally with live Tp. A control rabbit was not vaccinated prior to challenge. 105 Tp were injected at each of 8 sites along shaved rabbit backs. Black pen marks encircle each inoculation site.
4. Discussion
Using the gel microdroplet assay, we were unable to garner evidence that either TP0155 or TP0483 is surface-exposed in Tp [15]. Our conclusion that TP0155 and TP0483 likely are not outer membrane proteins seemingly contradicts the previous report by Cameron et al. [13] that TP0155 and TP0483 are fibronectin-binding proteins, based on observations that antibodies directed against TP0155 or TP0483 partially block the attachment of Tp to fibronectin-coated microscope slides. If TP0155 and TP0483 bind to fibronectin as part of Tp's dermal invasion strategy [18], then they should be outer membrane proteins with surface-exposed domains. However, TP0155, TP0483, or polypeptides with similar molecular masses have not been prominently noted in isolated Tp outer membranes [19], although their low abundance could explain a failure to detect them. Indeed, freeze-fracture freeze-etching electron microscopy of Tp has shown a paucity of proteins within treponemal outer membranes [20]. Furthermore, despite using high-titer rabbit antiserum directed against recombinant TP0155, we were unable to conclusively identify a protein of the correct molecular mass within whole-cell lysates of Tp, even though the rat-derived antibodies reacted with Tp in gel microdroplet assays. This discrepancy may reflect differences between Freund's adjuvant (used to generate rat antisera) and the Ribi adjuvant system (used for rabbit immunizations) [21]. Recent microarray data support the low abundance of TP0155 and TP0483, inasmuch as the ratios of mRNA-derived cDNA to DNA for both proteins are very low (0.333 for TP0155; 0.446 for TP0483) [17]. Finally, we were not able to demonstrate any protective effect of vaccinating rabbits with either TP0155 or TP0483, despite achieving high levels of serum antibodies against each protein after immunization.
The gel microdroplet assays also failed to indicate that TP0326 is surface-exposed in Tp. This finding contradicts protein homology searches [5] that assign TP0326 as a member of a family of porins [22] important in the assembly of Gram-negative bacterial outer membranes [5, 22]. The issue of whether Tp produces porins has been controversial; although there have been reports of outer membrane porins in Tp [11], such reports have been refuted [23, 24]. Although unlikely, it remains possible that TP0326 is a porin that lacks surface-exposed epitopes. On the other hand, Cameron et al. [5] reported that antibodies directed against TP0326 could serve as opsonins for the enhanced phagocytocis of Tp by macrophages, suggesting that TP0326 is surface-exposed. Pursuant to this line of reasoning, we immunized rabbits with recombinant TP0326 and failed to observe any degree of immunoprotection when these vaccinated animals were challenged intradermally with viable Tp.
Whereas the gel microdroplet assay currently is the most useful approach for investigating the cell-surface exposure of spirochetal antigens [15], it should be acknowledged that there are certain limitations to the technique. For example, not a single outer surface protein of Tp yet has been definitively identified, and thus there is not an antibody against a known surface protein of Tp that can be used as a positive control in the gel microdroplet assay. Thus, the overall sensitivity of the assay is not well established, especially for Tp outer membrane proteins that may be in low abundance or have few surface-exposed epitopes. Of note, a monoclonal antibody (M131) directed against treponemal phosphorylcholine has been shown to be reactive in gel microdroplet assays with Tp [25]. Indeed, we have used the M131 successfully in the gel microdroplet assay (not shown), although, in our hands, only about 50% of agarose gel-encapsulated spirochetes label, and the labeling is punctuate. Thus, whereas M131 may serve as a useful qualitative positive control in the gel microdroplet assay, its quantitative value in this assay remains unknown.
Although TP0956 has not been well studied, a Conserved Domain Database (CDD) search revealed that TP0956 is included within a family of 6 predicted integral membrane proteins (α-helical, not β-barrels) with unknown function. TP0956 has the highest identity (42%) with a probable lipoprotein in Treponema denticola (TDE1021) [26]. In our studies, antibodies directed against TP0956 and used in chemiluminescent immunoblots detected a polypeptide of the expected mass within whole-cell lysates of Tp, suggesting that TP0956 is readily expressed by Tp. The same antibody preparation, however, failed to react with Tp encapsulated in agarose gel microdroplets, even when mild detergent was used to disrupt the Tp outer membrane. The negative gel microdroplet results for TP0956 were surprising given that, as noted earlier, an antibody directed against TP0155 readily detected spirochetes in gel microdroplet assays but failed to detect its cognate antigen in chemiluminescent immunoblots. Although the reasons for positive reactivity of polyclonal antiserum against TP0956 in immunoblots but not in fluorescence gel microdroplet assays with Tp remain obscure, it is possible that recombinant protein immunization of rats elicited antibodies only to linear epitopes of the protein (plausible considering the protein purification techniques used), rather than to conformational or buried epitopes that might be more relevant to the actual cellular environment of TP0956. The notion of conformational-specific epitopes in Tp is not limited to TP0956 [27], and reports from other pathogenic bacteria support this possibility [28].
TP0956 also was previously hypothesized by McKevitt et al. to be a syphilis vaccine candidate [14]. This prediction was based on the correlation that chancre immunity in Tp–infected rabbits occurs between 60-120 days, and that the antibody response to TP0956 increases 30-fold at days 56-84 [14]. Thus, we proceeded with rabbit vaccination experiments. As with the other target antigens of this study, recombinant TP0956 failed to induce immunoprotection in the rabbit model of experimental syphilis.
Two additional aspects of our rabbit vaccination experiments are noteworthy. First, we remain cognizant that no single protein or lipoprotein may serve as a fully protective immunogen against Tp infection. For that reason, and recognizing that a degree of “partial protection” induced by any one immunogen may not be readily discernable in the rabbit model of experimental syphilis, we also immunized animals with a multivalent vaccine consisting of all four immunogens (TP0155, TP0326, TP0483, and TP0956). Although recently a multivalent vaccine has shown promise for immunoprotection against Staphylococcus aureus using four surface proteins [29], our multivalent vaccine failed to induce protective immunity in rabbits. Second, Cameron et al. [5] previously observed a decrease in the severity of rabbit dermal lesions after rabbits immunized with recombinant TP0326 were intradermally challenged with viable treponemes [5]. In our study, after comparable vaccination with recombinant TP0326, all rabbits developed ulcerated lesions. This result occurred despite our efforts to follow precisely the vaccination and challenge protocol of Cameron et al. [5]. On the other hand, any number of subtle parameters could have influenced our differing results, including the fact that New Zealand rabbits are outbred. Performing vaccine trials with results that are consistent from laboratory to laboratory (using the rabbit model of experimental syphilis) remains one of the formidable technical challenges in syphilis vaccine research. Perhaps more contemporary vaccinology strategies, such as genetic immunization, may help reduce potential variables such as protein misfolding, conformational epitopes, or ineffective protein processing/presentation.
The quest to define the molecular component(s) of Tp that might induce protective immunity in rabbits should not be daunted. While the search for a bona fide surface-exposed OMP remains paramount, it is also arguable that a better understanding, in general, of syphilis immunology, particularly as it pertains to the development of chancre immunity in rabbits, can offer new insights into elucidating previously unknown immunological aspects of chronic bacterial infections. It is plausible that both humoral and cell-mediated immune mechanisms contribute to the clearance (resolution of lesions) and control (latency) of treponemal infection [30, 31], and clarifying the roles of both of these arms of the immune system remains warranted.
5. Acknowledgments
The authors thank Martin Goldberg for expert technical assistance, David L. Cox for training and advice on the gel microdroplet technique, and David R. Blanco for the M131 monoclonal antibody.
This work was supported by a grant from the National Institute of Allergy and Infectious Diseases, National Institutes of Health (R01-AI016692). F.L.T. was supported by the National Institutes of Health Training Grant (T32-AI007520).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gerbase AC, Rowley JT, Heymann DH, Berkley SF, Piot P. Global prevalence and incidence estimates of selected curable STDs. Sexually transmitted infections. 1998;74(Suppl 1):S12–16. [PubMed] [Google Scholar]
- 2.Sellati TJ, Wilkinson DA, Sheffield JS, Koup RA, Radolf JD, Norgard MV. Virulent Treponema pallidum, lipoprotein, and synthetic lipopeptides induce CCR5 on human monocytes and enhance their susceptibility to infection by human immunodeficiency virus type 1. J Infect Dis. 2000;181:283–293. doi: 10.1086/315209. [DOI] [PubMed] [Google Scholar]
- 3.Turner TB, Hollander DH. Biology of the treponematoses based on studies carried out at the International Treponematosis Laboratory Center of the Johns Hopkins University under the auspices of the World Health Organization. Monogr Ser World Health Organ. 1957:3–266. [PubMed] [Google Scholar]
- 4.Miller JN. Immunity in experimental syphilis. VI. Successful vaccination of rabbits with Treponema pallidum, Nichols strain, attenuated by -irradiation. J Immunol. 1973;110:1206–1215. [PubMed] [Google Scholar]
- 5.Cameron CE, Lukehart SA, Castro C, Molini B, Godornes C, Van Voorhis WC. Opsonic potential, protective capacity, and sequence conservation of the Treponema pallidum subspecies pallidum Tp92. J Infect Dis. 2000;181:1401–1413. doi: 10.1086/315399. [DOI] [PubMed] [Google Scholar]
- 6.Cullen PA, Cameron CE. Progress towards an effective syphilis vaccine: the past, present and future. Expert Rev Vaccines. 2006;5:67–80. doi: 10.1586/14760584.5.1.67. [DOI] [PubMed] [Google Scholar]
- 7.Magnuson HJ, Thomas EW, Olansky S, Kaplan BI, De Mello L, Cutler JC. Inoculation syphilis in human volunteers. Medicine (Baltimore) 1956;35:33–82. doi: 10.1097/00005792-195602000-00002. [DOI] [PubMed] [Google Scholar]
- 8.Sell S, Norris SJ. The biology, pathology, and immunology of syphilis. Int Rev Exp Pathol. 1983;24:203–276. [PubMed] [Google Scholar]
- 9.Magnuson H, BJ R. The rate of development and degree of acquired immunity in experimental syphilis. Am. J. Syph. Gon. Vener. Dis. 1948;32:418–436. [PubMed] [Google Scholar]
- 10.Radolf JD. Treponema pallidum and the quest for outer membrane proteins. Mol Microbiol. 1995;16:1067–1073. doi: 10.1111/j.1365-2958.1995.tb02332.x. [DOI] [PubMed] [Google Scholar]
- 11.Blanco DR, Miller JN, Lovett MA. Surface antigens of the syphilis spirochete and their potential as virulence determinants. Emerg Infect Dis. 1997;3:11–20. doi: 10.3201/eid0301.970102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Norris SJ. Polypeptides of Treponema pallidum: progress toward understanding their structural, functional, and immunologic roles. Treponema Pallidum Polypeptide Research Group. Microbiol Rev. 1993;57:750–779. doi: 10.1128/mr.57.3.750-779.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cameron CE, Brown EL, Kuroiwa JM, Schnapp LM, Brouwer NL. Treponema pallidum fibronectin-binding proteins. J Bacteriol. 2004;186:7019–7022. doi: 10.1128/JB.186.20.7019-7022.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McKevitt M, Brinkman MB, McLoughlin M, Perez C, Howell JK, Weinstock GM, Norris SJ, Palzkill T. Genome scale identification of Treponema pallidum antigens. Infect Immun. 2005;73:4445–4450. doi: 10.1128/IAI.73.7.4445-4450.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cox DL, Akins DR, Porcella SF, Norgard MV, Radolf JD. Treponema pallidum in gel microdroplets: a novel strategy for investigation of treponemal molecular architecture. Mol Microbiol. 1995;15:1151–1164. doi: 10.1111/j.1365-2958.1995.tb02288.x. [DOI] [PubMed] [Google Scholar]
- 16.Jones SA, Marchitto KS, Miller JN, Norgard MV. Monoclonal antibody with hemagglutination, immobilization, and neutralization activities defines an immunodominant, 47,000 mol wt, surface-exposed immunogen of Treponema pallidum (Nichols) J Exp Med. 1984;160:1404–1420. doi: 10.1084/jem.160.5.1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smajs D, McKevitt M, Howell JK, Norris SJ, Cai WW, Palzkill T, Weinstock GM. Transcriptome of Treponema pallidum: gene expression profile during experimental rabbit infection. J Bacteriol. 2005;187:1866–1874. doi: 10.1128/JB.187.5.1866-1874.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee JH, Choi HJ, Jung J, Lee MG, Lee JB, Lee KH. Receptors for Treponema pallidum attachment to the surface and matrix proteins of cultured human dermal microvascular endothelial cells. Yonsei Med J. 2003;44:371–378. doi: 10.3349/ymj.2003.44.3.371. [DOI] [PubMed] [Google Scholar]
- 19.Radolf JD, Robinson EJ, Bourell KW, Akins DR, Porcella SF, Weigel LM, Jones JD, Norgard MV. Characterization of outer membranes isolated from Treponema pallidum, the syphilis spirochete. Infect Immun. 1995;63:4244–4252. doi: 10.1128/iai.63.11.4244-4252.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Radolf JD, Norgard MV, Schulz WW. Outer membrane ultrastructure explains the limited antigenicity of virulent Treponema pallidum. Proc Natl Acad Sci U S A. 1989;86:2051–2055. doi: 10.1073/pnas.86.6.2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Deeb BJ, DiGiacomo RF, Kunz LL, Stewart JL. Comparison of Freund’s and Ribi adjuvants for inducing antibodies to the synthetic antigen (TG)-AL in rabbits. J Immunol Methods. 1992;152:105–113. doi: 10.1016/0022-1759(92)90093-9. [DOI] [PubMed] [Google Scholar]
- 22.Yen MR, Peabody CR, Partovi SM, Zhai Y, Tseng YH, Saier MH. Protein-translocating outer membrane porins of Gram-negative bacteria. Biochim Biophys Acta. 2002;1562:6–31. doi: 10.1016/s0005-2736(02)00359-0. [DOI] [PubMed] [Google Scholar]
- 23.Akins DR, Robinson E, Shevchenko D, Elkins C, Cox DL, Radolf JD. Tromp1, a putative rare outer membrane protein, is anchored by an uncleaved signal sequence to the Treponema pallidum cytoplasmic membrane. J Bacteriol. 1997;179:5076–5086. doi: 10.1128/jb.179.16.5076-5086.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Deka RK, Lee YH, Hagman KE, Shevchenko D, Lingwood CA, Hasemann CA, Norgard MV, Radolf JD. Physicochemical evidence that Treponema pallidum TroA is a zinc-containing metalloprotein that lacks porin-like structure. J Bacteriol. 1999;181:4420–4423. doi: 10.1128/jb.181.14.4420-4423.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Blanco DR, Champion CI, Dooley A, Cox DL, Whitelegge JP, Faull K, Lovett MA. A monoclonal antibody that conveys in vitro killing and partial protection in experimental syphilis binds a phosphorylcholine surface epitope of Treponema pallidum. Infect Immun. 2005;73:3083–3095. doi: 10.1128/IAI.73.5.3083-3095.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marchler-Bauer A, Anderson JB, Cherukuri PF, DeWeese-Scott C, Geer LY, Gwadz M, He S, Hurwitz DI, Jackson JD, Ke Z, Lanczycki CJ, Liebert CA, Liu C, Lu F, Marchler GH, Mullokandov M, Shoemaker BA, Simonyan V, Song JS, Thiessen PA, Yamashita RA, Yin JJ, Zhang D, Bryant SH. CDD: a Conserved Domain Database for protein classification. Nucleic Acids Res. 2005;33:D192–196. doi: 10.1093/nar/gki069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.LaFond RE, Molini BJ, Van Voorhis WC, Lukehart SA. Antigenic variation of TprK V regions abrogates specific antibody binding in syphilis. Infect Immun. 2006;74:6244–6251. doi: 10.1128/IAI.00827-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wolf K, Fischer E, Mead D, Zhong G, Peeling R, Whitmire B, Caldwell HD. Chlamydia pneumoniae major outer membrane protein is a surface-exposed antigen that elicits antibodies primarily directed against conformation-dependent determinants. Infect Immun. 2001;69:3082–3091. doi: 10.1128/IAI.69.5.3082-3091.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stranger-Jones YK, Bae T, Schneewind O. Vaccine assembly from surface proteins of Staphylococcus aureus. Proc Natl Acad Sci U S A. 2006;103:16942–16947. doi: 10.1073/pnas.0606863103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sellati TJ, Waldrop SL, Salazar JC, Bergstresser PR, Picker LJ, Radolf JD. The cutaneous response in humans to Treponema pallidum lipoprotein analogues involves cellular elements of both innate and adaptive immunity. J Immunol. 2001;166:4131–4140. doi: 10.4049/jimmunol.166.6.4131. [DOI] [PubMed] [Google Scholar]
- 31.Salazar JC, Hazlett KR, Radolf JD. The immune response to infection with Treponema pallidum, the stealth pathogen. Microbes Infect. 2002;4:1133–1140. doi: 10.1016/s1286-4579(02)01638-6. [DOI] [PubMed] [Google Scholar]