Abstract
The main purpose of the present study was to determine whether specific regions of the mouse brain exhibit different age-related changes in oxidative stress, as indicated by glutathione redox state and the level of protein-glutathionyl mixed disulfides. Comparison of 3- and 21-month-old mice indicated an age-related decrease in the ratio of reduced to oxidized glutathione (GSH:GSSG) as well as a pro-oxidizing shift in the calculated redox potential (ranging from 6 to 15 mV) in the cortex, hippocampus, striatum and cerebellum, whereas there was little change in the brainstem. This pro-oxidizing shift in redox state was due to a modest decrease in GSH content occurring in all the brain regions examined, and elevations in GSSG amount that were most pronounced in the striatum and cerebellum. The regional changes in glutathione redox state were paralleled by increases in the amounts of protein-mixed disulfides. A reduction of caloric intake by 40% for a short period (7 weeks), implemented in relatively old mice (17 months), increased the GSH:GSSG ratio and redox potential at 19 months in the same brain regions that exhibited age-related decreases. The effects of age and caloric restriction were qualitatively similar in C57BL/6 and DBA/2 mice. However, young DBA/2 mice, which do not show extension of life span in response to long-term caloric restriction, had lower GSH:GSSG ratios and higher protein-mixed disulfides than age-matched C57BL/6 mice. The current findings demonstrate that oxidative stress, as reflected by glutathione redox state, increases in the aging brain in regions linked to age-associated losses of function and neurodegenerative diseases.
Keywords: Glutathione, protein-mixed disulfides, redox potential, caloric restriction, inbred mice, aging, cerebral cortex, hippocampus, striatum, cerebellum, brainstem
1. Introduction
Losses in cognition and psychomotor functions are common manifestations of senescence in mammals, however, the onset and severity of such losses seem to be relatively independent of each other, suggesting that different regions of the brain may undergo senescence at different rates (Gage et al., 1989; Collier and Coleman, 1991; Forster et al., 1996; Markowska and Breckler, 1999). Although the mechanisms responsible for age-related functional deteriorations are not well understood, accrual of macromolecular oxidative damage is widely postulated to play a causal role. Indeed, amounts of oxidatively modified proteins, lipids and nucleic acids differentially increase with age in discrete regions of the brain (reviewed by Floyd and Hensley, 2002; Barja, 2004), as do patterns of gene expression suggestive of oxidative stress (Lee et al., 2000). Protein carbonyl content, a measure of protein oxidation, increases with age most rapidly in hippocampus and striatum (Dubey et al., 1996), regions that are associated with significant losses in function during aging. This increase is attenuated by restriction of caloric intake (Dubey et al., 1996; Forster et al., 2000), a regimen that prolongs life span of rodents (Sohal and Weindruch, 1996) and reverses age-associated gene expression (Lee et al., 2000).
In addition to accrual of oxidative damage, the redox state of tissues shifts towards a more oxidant state during aging (Rebrin et al., 2003). The redox state is determined by the amounts and ratios of interconvertable forms of redox couples such as GSH/GSSG, NADPH/NADP+, and thioredoxinred/ thioredoxinox, among others (Schafer and Buettner, 2001). Because glutathione is 2- to 3 orders of magnitude more abundant than the others, it is considered to be a major indicator of the overall redox state. One consequence of oxidation of GSH and/or protein cysteinyl residues is the uncatalyzed formation of protein-glutathionyl mixed disulfides. This reversible interaction has been postulated to serve as a regulatory mechanism for proteins involved in a variety of functions such as biosignaling, gene transcription, and catalytic activities (Cotgreave and Gerdes, 1998; Droge, 2002). An age-associated, pro-oxidizing shift in glutathione redox state and an elevation of protein mixed disulfides (protein-SSG) have been previously reported in Drosophila melanogaster (Rebrin et al., 2004) and different tissues of C57BL/6 mice (Rebrin et al., 2003). Using the Nernst equation, amounts of GSH and GSSG can be used to calculate the redox potential of the tissues, considered to be a measure of oxidative stress (Schafer and Buettner, 2001; Jones, 2002; Rebrin et al., 2003).
The main purpose of this study was to determine whether regions of the mouse brain differ in the effect of age on redox state and whether caloric restriction (CR) could modify such changes. To assess age-related changes, comparisons were made between ad libitum fed 3- and 21-month old C57BL/6 and DBA/2 mice, whereas the effects of CR were determined in 19-month-olds of these strains. Comparison of the C57BL/6 and DBA/2 mice was made because these strains differ markedly in their response to CR implemented at different ages. When initiated at maturity and maintained throughout life, CR prolonged the life span of C57BL/6 mice, but not of the DBA/2 mice (Forster et al., 2003). When implementation was delayed until older ages, CR had no effect on the mortality of C57BL/6 mice, but markedly accelerated the mortality rate of DBA/2 mice. Independent of its effects on mortality, the same late-life CR regimen resulted in improvements in psychomotor function of the C57BL/6 mice but not of the DBA/2 mice (Forster and Lal, 1999). Therefore, an additional goal of this study was to determine if the different responses of these strains to late-life CR were correlated with changes in the glutathione redox state.
2. Results
2.1. Age-related changes in glutathione redox state and protein-SSG
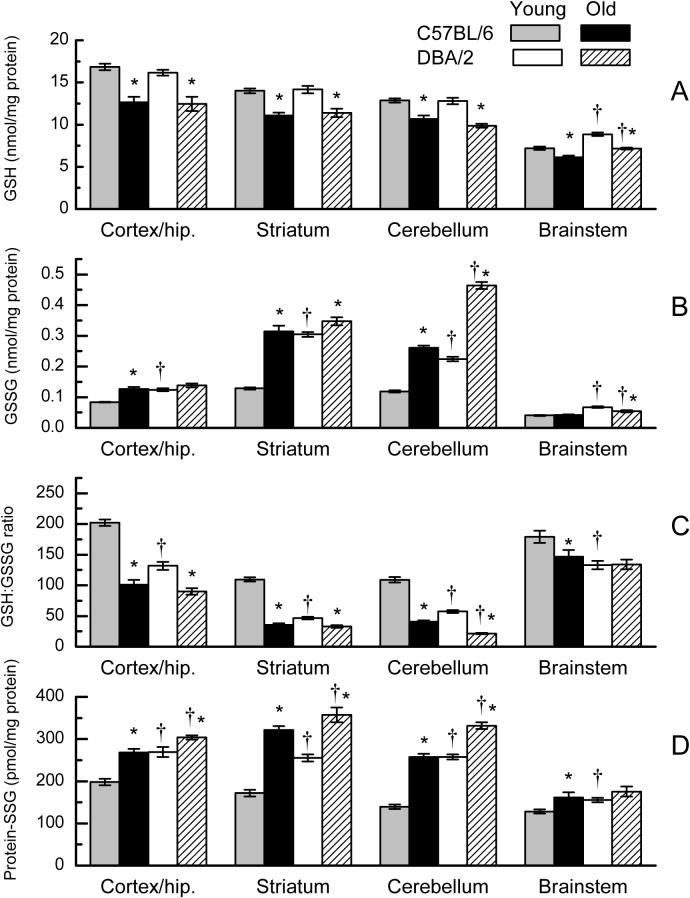
The amounts of GSH, GSSG and protein-SSG were measured in cortex/hippocampus, striatum, cerebellum and brain stem of 3- and 21-month-old C57BL/6 and DBA/2 mice for the purpose of determining the effects of age and genetic background on glutathione redox state in different regions of the brain. In general, there was considerable regional variation in the distribution as well as the magnitude of age-related changes in the amounts of GSH, GSSG and protein-SSG. Overall, the concentration of GSH varied about 2-fold in different brain regions, with the rank order: cortex/hippocampus > striatum > cerebellum > brainstem (Fig. 1A). There was a significant decrease (15−25%) in GSH level with age in all of the brain regions in both strains of mice. The two strains did not differ in GSH level at any age in the cortex/hippocampus, striatum, or cerebellum, although the GSH amount was ∼ 20% higher in the brainstem of DBA/2 mice when compared with age-matched C57BL/6 mice. The 3-way analysis of variance of GSH content indicated significant main effects of Age and Brain Region (p < 0.001) as well as a significant interaction of Region with Strain (p = 0.005), but did not indicate any significant interactions involving Strain and Age. A similar analysis of variance conducted on the protein content of the same tissues did not indicate a significant effect of Age, Strain, or the interaction of those factors (all p>0.08).
Fig. 1.
GSH (A), GSSG (B), GSH:GSSG ratio (C), and protein-SSG (D) in homogenates of different regions of brain in young (3 months) and old (21 months) C57BL/6 and DBA/2 mice. Data represent the mean ± SE of 5−8 mice. (* p<0.05 for old vs young; † for C57BL/6 vs DBA/2; based on F tests within Age x Strain x Region interaction).
The GSSG content varied markedly as a function of age, strain and brain region. In young C57BL/6 mice, amounts of GSSG were highest in striatum and cerebellum, with progressively lower amounts in cortex/hippocampus and brainstem (Fig. 1B). In the same brain regions of young mice, the GSSG concentration was markedly higher in DBA/2 mice than in C57BL/6 mice, e.g., 140% in the striatum, 89% in the cerebellum, 49% in the cortex/hippocampus and 65% in the brainstem. Both strains showed > 120% age-related elevation in GSSG content in the cerebellum. However, in striatum and cortex/hippocampus, age-related increases were more prominent in the C57BL/6 mice (150% in striatum, 52% in cortex/hippocampus) than in the DBA/2 mice (< 15%). Age had no affect on GSSG amount in the brainstem of C57BL/6 mice, whereas there was a 20% decrease in the DBA/2 mice. The analysis of variance on GSSG amounts indicated significant main effects of Age, Strain, and Brain Region (p < 0.001) as well as a significant 3-way interaction among these factors (p < 0.001).
The pattern of GSH:GSSG ratio in different brain regions was similar in the young DBA/2 and C57BL/6 mice, with the relatively highest ratios present in cortex/hippocampus and brainstem and lower ratios in striatum and cerebellum (Fig. 1C). However, in accord with the difference in GSSG content among the two mouse strains, the GSH:GSSG ratio in the young mice was 26 to 57% lower within specific regions of the brain in DBA/2 mice, compared with C57BL/6 mice. Age-related decreases in GSH:GSSG ratios occurred in the cerebellum, cortex/hippocampus, and striatum of both strains. For instance, in C57BL/6 and DBA/2 mice the declines were, respectively, 63% and 63% in the cerebellum, 50% and 29% in cortex/hippocampus, and 67% and 32% in striatum. There was an 18% age-related decrease in GSH:GSSG ratio in the brainstem of C57BL/6 mice, whereas there was no significant change evident in DBA/2 mice. The analysis of variance on GSH:GSSG ratio indicated significant main effects of Age, Strain, and Brain Region (p < 0.001) and a significant interaction of Strain with Age (p < 0.001).
The variations in amounts of protein-SSG (Fig. 1D) in different brain regions of young C57BL/6 mice paralleled the concentration of GSH, with the rank order: cortex/hippocampus > striatum > cerebellum > brainstem. Compared with C57BL/6 mice at 3-month of age, the protein-SSG concentration was higher in DBA/2 mice by 36-, 49-, 85-, and 21%, respectively, in the cortex/hippocampus, striatum, cerebellum, and brainstem. Age-related increases in protein-SSG content occurred in all brain regions of C57BL/6 mice, most markedly in the cerebellum (85%) and striatum (87%) and to a lesser extent in cortex/hippocampus (35%) and brainstem (26%). Smaller, though statistically significant, age-related increases were also evident in the striatum (40%), cerebellum (29%) and cortex/hippocamupus (13%) of DBA/2 mice. Analysis of variance of protein SSG indicated significant main effects of Age, Strain, and Brain Region (p < 0.001) and indicated significant 2-way interactions of Strain with Age and Brain Region (p < 0.001).
2.2. Effect of caloric restriction on glutathione redox state and protein-SSG content
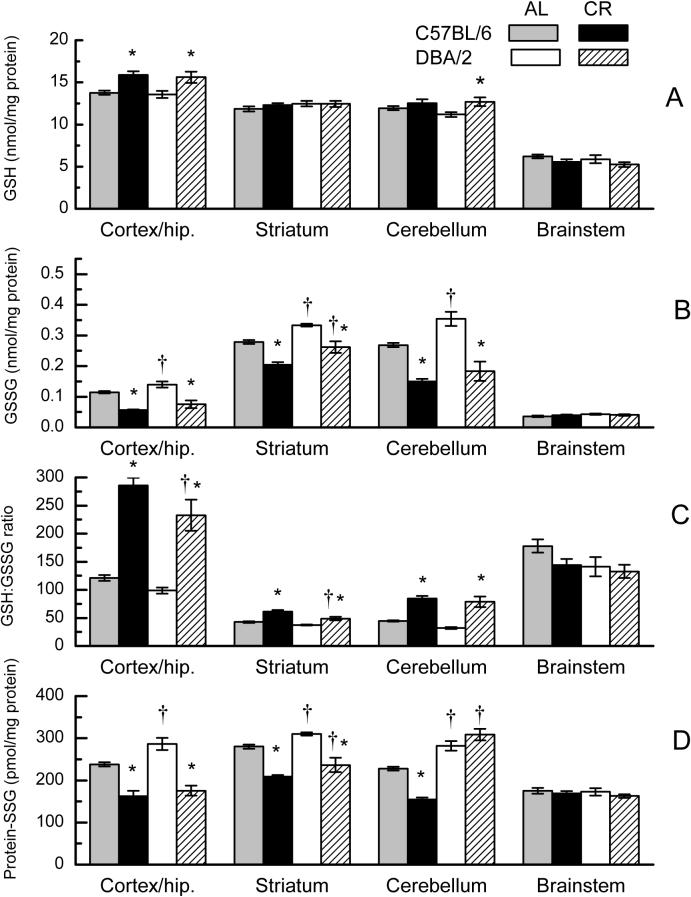
Groups of ad libitum (AL) fed C57BL/6 and DBA/2 mice were transferred at 17 months of age to a regimen of 40% caloric restriction for 7 weeks for the purpose of determining whether short-term CR affects the age-related shifts in redox state of glutathione and accrual of protein-SSG (Fig. 2A-D). When measured in these mice at 19 months of age, the relative amounts of GSH in different regions of the brain in the AL group of each strain paralleled those shown in Figure 1A for 21-month-old mice. In the CR groups, GSH amounts showed a 15% increase in the cortex/hippocampus of both DBA/2 and C57BL/6 mice, and a 14% increase in the cerebellum of DBA/2 mice (Fig. 2A). Caloric restriction did not affect GSH content of either strain in the striatum or brainstem. The effect of CR on cortex/hippocampus and cerebellum, but not on other brain regions, was reflected in a significant interaction of Diet with Brain Region (p < 0.001). The main effects and interactions involving Strain were not significant (p > 0.480).
Fig. 2.
GSH (A), GSSG (B), GSH:GSSG ratio (C), and protein-SSG (D) in homogenates of different regions of the brain in 19-month-old C57BL/6 and DBA/2 mice after 7 weeks on AL or CR diets. Data represent the mean ± SE of 5−8 mice. (* p<0.05 for AL vs CR; † for C57BL/6 vs DBA/2; based on F tests within Strain x Diet x Region interaction).
In both strains of mice, CR resulted in a significant decrease in GSSG content in all of the brain regions except the brainstem (Fig. 2B). The relative magnitude of the decreases was similar in the C57BL/6 and DBA/2 mice for the cortex/hippocampus (51%, 46%), striatum (27%, 22%) and cerebellum (44%, 48%). The decrease in GSSG amount following CR in these regions was paralleled by an increase in GSH:GSSG ratio (Fig. 2C). In both strains of mice, the increases were most marked in the cortex/hippocampus (140% in both strains) and cerebellum (90% in C57BL/6, 150% in DBA/2), and were of a lesser magnitude in the striatum (43% in C57BL/6, 30% in DBA/2). The GSH:GSSG ratio of the brainstem was not significantly affected by the CR regimen. Analyses of variance of GSSG as well as GSH:GSSG ratio showed significant main effects of Strain, Diet and Region (p < 0.002), as well as a Region x Diet interaction (p < 0.001). Neither analysis revealed a significant interaction of Strain with Diet (p > 0.194).
In response to CR, protein-SSG content was found to decrease by 25 to 32% in the cortex/hippocampus, striatum, and cerebellum of the C57BL/6 mice (Fig. 2D). A similar decrease in protein-SSG amount (24−39%) was evident in the cortex/hippocampus and striatum of DBA/2 mice. However, the protein-SSG content in the cerebellum of DBA/2 mice did not show a significant decline following CR. There was no apparent effect of CR on protein-SSG amounts in the brainstem in either of the two strains of mice. The absence of an effect of CR on protein-SSG in DBA/2 mice, evident only in the cerebellum, contributed to a significant 3-way interaction of Strain, Diet and Brain Region (p > 0.001).
2.3. Effect of genotype, age and caloric intake on glutathione redox potential
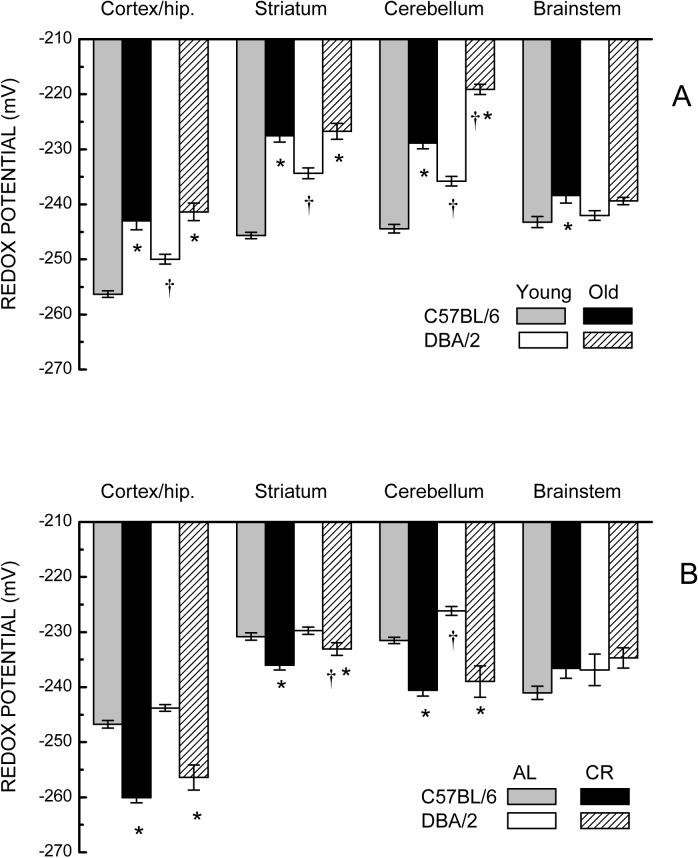
The glutathione redox potential of homogenates of different brain regions of the two mouse strains was calculated using the Nernst equation and the amounts of GSH and GSSG presented in Figures 1 and 2. A comparison of glutathione redox potential in young mice had the rank order: cortex/hippocampus > striatum ≈ cerebellum ≈ brainstem for C57BL/6 mice, and cortex/hippocampus > brainstem > cerebellum ≈ striatum for the DBA/2 mice (Fig. 3). The redox potential was higher in the young C57BL/6 than young DBA/2 mice in three regions, namely cortex/hippocampus, striatum and cerebellum by 6−12 mV, but did not appreciably differ in the brainstem. Old C57BL/6 and DBA/2 mice exhibited marked decreases (8−18 mV) in redox potential in the cortex/hippocampus, striatum and cerebellum, but relatively small shifts in the brainstem (3−4 mV). A three-way ANOVA on redox potential confirmed a significant 3-way interaction of Age, Strain, and Brain Region (p=0.001).
Fig. 3.
(A) Effect of age on glutathione redox potential (mV± SE) in homogenates of different brain regions (left to right) in C57BL/6 and DBA/2 mice. (* p<0.05 for old vs young; † for C57BL/6 vs DBA/2; based on F tests within Age x Strain x Region interaction). (B) Glutathione redox potential in homogenates of different brain regions in 19-month-old C57BL/6 and DBA/2 mice after 7 weeks on AL or CR diets. (* p<0.05 for AL vs CR; † for C57BL/6 vs DBA/2; based on F tests within Strain x Diet x Region interaction).
The region-specific, age-related decreases in redox potential were fully or partially reversed following 7 weeks of CR (Fig. 3B). In cortex/hippocampus and cerebellum, the redox potential of CR groups in both strains was equivalent or greater than that of their younger counterparts (see Fig. 3A). Somewhat smaller, though significant effects of CR were evident in the striatum of both mouse strains, while CR did not have an apparent effect on redox potential of the brainstem. Analyses of redox potential data indicated significant main effects of Diet and Region, as well as a Region by Diet interaction (p<0.001), but did not indicate significant interactions involving Diet and Strain (p>0.468).
4. Discussion
The main findings of this study were that: (i) The amounts of GSH, GSSG and protein-SSG content were notably greater in tissue homogenates from areas of the forebrain (cortex, hippocampus, striatum) and cerebellum than in the brainstem, in both young and old mice of the two different strains. (ii) The GSH:GSSG ratios decreased significantly with age in the forebrain regions and cerebellum in both strains of mice, whereas there was little or no change in the brainstem. The age-related decreases in GSH:GSSG ratio were attributable to both a modest decrease in GSH content, which occurred uniformly in all regions examined, and increases in GSSG amount, which were most pronounced in the striatum and cerebellum. (iii) The age-related decreases in GSH:GSSG ratio of the different brain regions were paralleled by proportional increases in protein-SSG. (iv) Young DBA/2 mice had lower GSH:GSSG ratios than age-matched C57BL/6 mice in all brain regions, and had relatively higher concentrations of protein-SSG, matching the concentrations of the old C57BL/6 mice. (v) A reduction of caloric intake by 40% for a period of only 7 weeks increased the GSH:GSSG ratio of old mice, primarily by decreasing amounts of GSSG in regions of the forebrain and cerebellum. (vi) Reduced caloric intake in old mice resulted in a marked decrease of protein-SSG content in regions of the forebrain for both mouse strains, but in the cerebellum it occurred only in the C57BL/6 mice.
The structural and functional heterogeneity of the different brain regions and their differential susceptibility to aging and degenerative diseases has been well established. For example, losses of certain cognitive functions, in both aging and in Alzheimer's disease, involve the cerebral cortex, cortical inputs to the hippocampus, and the hippocampus proper (Hyman et al., 1984; Hof et al., 1990; Geinisman et al., 1995; Morrison and Hof, 1997). On the other hand, losses of psychomotor function have been linked to alterations in the striatum (Joseph and Roth, 1988; Chesselet and Delfs, 1996; Volkow et al., 1998) and cerebellum (Forster et al., 1996; Uecker et al., 2000). Results of this study indicate that the level of oxidative stress is increased markedly with age in these disease-vulnerable regions. For instance, the magnitude of the age-related decrease in GSH:GSSG ratio and pro-oxidizing shift in the calculated redox potential, as well as the increase in protein-SSG content, was greatest in the striatum, cerebellum, and cortex/hippocampus; whereas the effect of age was relatively small in the brainstem, a region not commonly implicated in age-associated behavioral impairments. Previous studies on age-associated oxidative damage yielded a similar pattern of changes. Dubey et al. (1996) reported age-related increases in protein oxidation in several regions of the forebrain, most notably in the hippocampus and striatum, whereas no change was observed in the brainstem. Such oxidative damage to proteins would seem to have functional implications, as suggested by the correlation of the severity of the damage with cognitive or motor performance of aged mice or rats (Forster et al., 1996; Nicolle et al., 2001).
Results of the present study indicated that most brain regions express a moderate age-related depletion of GSH content (about 15−25%), whereas marked increases in GSSG occurred only in the striatum and cerebellum. Depletion of GSH has been reported in some, but not all the tissues of aged mice and rats (Liu and Choi, 2000; Liu, 2002; Rebrin et al., 2003; Wang et al., 2003). However, in the brain, a loss of GSH seems to be a consistent phenomenon (Wang et al., 2003; Suh et al., 2004) that may be the result of a concomitant age-related decrease in the activity of glutamate cysteine ligase, a rate-limiting enzyme in de novo synthesis of GSH. Since the loss of GSH in different brain regions was of relatively similar magnitude in the current and in previous studies of male C57BL/6 mice (Wang et al., 2003), a regional difference in GSH concentration, resulting from impaired biosynthesis, cannot readily account for the regional differences in GSH:GSSG ratio, redox potential or protein SSG in the aged mice. The regional differences may instead reflect different levels of oxidative stress as a result of increased generation of reactive oxygen species (ROS), as reported previously (Sohal et al., 1994), or age-related regional differences in the metabolic fate of GSSG.
Disturbance in redox homeostasis is likely to have a variety of deleterious functional consequences. Depletion of GSH would tend to attenuate the antioxidant capacity of cells. For instance, GSH can react directly with ROS to form thiyl radical which, following a series of transformations, including autoxidation, leads to the generation of superoxide and its elimination by the activity of superoxide dismutase. Thus GSH and SOD, acting in concert, lower the steady state oxidant load of the cells (Winterbourn, 1995). GSH also provides, directly or secondarily, reducing equivalents for the elimination of peroxides. In addition, GSH forms protein mixed disulfides with thiyl radicals of protein cysteinyl residues (Wardman, 1995; Cotgreave and Gerdes, 1998; Droge, 2002). This reaction is reversible and affords protection for protein thiols against further oxidation, and may act as a mechanism for the redox-based regulation of biosignaling and gene transcription. In this context, the observed deterioration in glutathione redox state can be predicted to have various physiological effects including an enhanced production of ROS (e.g., Aoyama et al., 2006), decreased glutathione S-transferase-mediated elimination of electrophilic xenobiotics and/or the end-products of lipid peroxidation, and alterations in thresholds for activation/inactivation of redox-sensitive proteins, some involved in neural signaling cascades important for memory and other brain processes (Serrano and Klann, 2004).
An important finding of this study is that the implementation of CR for only 7 weeks in relatively old mice, after they had been fed ad libitum for 17 months, produced a reversal of age-related changes in the GSH:GSSG ratio and redox potential in the very same regions that showed age-related decreases in mice fed ad libitum continuously. A similar pattern of results has been observed in studies on the effect of short-term CR on gene expression (Spindler, 2005) and protein oxidation (Dubey et al., 1996; Forster et al., 2000). Whereas accrual of protein carbonyls was slowed by long-term CR, a similar, albeit attenuated, effect could be achieved after only 6 weeks of CR in mice of matching age. Conversely, a discontinuation of the long-term CR regimen in 20-month-old mice resulted in an increase in carbonyl content, to the level of the age-matched AL controls, within one week. The present findings are in accordance with the previous results and additionally suggest that these apparently reversible effects of CR on oxidative damage relate to a direct effect on the level of cellular oxidative stress.
The use of two different strains of mice in the current study allowed an assessment of the generality of the effect of age and CR on the glutathione redox state as well as the relationship between brain glutathione redox state and certain phenotypes peculiar to DBA/2 mice, including insensitivity to the beneficial effects of CR (Forster and Lal, 1999; Forster et al., 2003), and a pattern of impaired spatial learning (Upchurch and Wehner, 1988; Kempermann and Gage, 2002) and psychomotor function (Forster and Lal, 1999) relative to C57BL/6 mice. The current results suggest that the two mouse strains do not differ with regard to the regional pattern or amounts of GSH, or in the effect of age on GSH:GSSG ratios and redox potential. However, young DBA/2 mice showed a markedly low GSH:GSSG ratio in all brain regions, comparable to the level present in the old C57BL/6 mice. The low GSH:GSSG ratios in young DBA/2 mice were due to relatively high amounts of GSSG and did not involve any major difference from C57BL/6 mice in GSH content. The higher amounts of GSSG in the brains of both the young and the old DBA/2, compared to C57BL/6 mice, indicate a relatively higher level of oxidative stress is maintained throughout life in this mouse strain. Although there are no previous reports comparing oxidative damage in brains of the C57BL/6 and DBA/2 strains, Gerhard et al. (Gerhard et al., 2002) have reported hepatic malondialdehyde concentration to be 50% higher in young DBA/2 when compared to C57BL/6. Together with the current results, such findings suggest that a chronically high level of oxidative stress may be involved in the phenotypes expressed by the DBA/2 mice.
It was expected that the ability of CR to affect the glutathione redox state might differ in the DBA/2 and C57BL/6 mice, based on the resistance of DBA/2 mice to the life-prolonging effect of CR, and the different effects of late-life CR on mortality and motor function in these strains (Forster and Lal, 1999; Forster et al., 2003). However, despite their relatively low GSH:GSSG ratios, the old DBA/2 mice did not differ markedly from C57BL/6 with regard to the ability of CR to increase the GSH:GSSG ratio and the calculated redox potential, or to decrease the amounts of GSSG in regions of the forebrain or cerebellum. These results suggest that the ineffectiveness of late-life CR in promoting improved motor function in DBA/2 mice is not attributable to any obvious strain difference in the effect of CR on oxidative stress.
To conclude, brain aging appears to involve a widening of the imbalance between pro-oxidants and antioxidants, as reflected by glutathione redox potential. This finding is consistent with gene expression profiles of the aging mouse brain suggesting increased oxidative stress (Lee et al., 2000), and provides further support, albeit circumstantial, of the concept that oxidative stress may be an underlying cause of brain dysfunction. The brain regions in which redox imbalance is most pronounced are linked to accrual of oxidative damage and associated losses of function, and are susceptible to neurodegenerative diseases.
4. Experimental procedures
4.1. Reagents
GSH and GSSG were obtained from Sigma Chemical Co. (St Louis, MO) and used as calibration standards. Acetonitrile, meta-phosphoric acid, and 1-octane sulfonic acid were from EM Science (Gibbstown, NJ). Deionized water was prepared using a Millipore Milli-Q System (Milli-Q Corp., Bedford, MA, USA). All other chemicals were HPLC grade or of the highest purity available.
4.2. Animals
Thirty male C57BL/6JNia and 25 male DBA/2JNia mice of different ages were obtained from the National Institute on Aging, National Institutes of Health and housed individually in 28 × 19 × 12.5 cm clear polycarbonate cages with wire tops at 23°±1°C, 40% relative humidity, and 12-h light/dark cycle with lights on at 0600 h. Mice had ad libitum access to food (NIH-31) and water or were maintained under 40% caloric restriction. Mice were weighed weekly. All procedures were approved by the relevant institutional committees of the University of Southern California and University of North Texas Health Science Center.
4.3. Caloric restriction
Seventeen-month-old C57BL/6 and DBA/2 mice were assigned to ad libitum feeding (AL) or caloric restriction (CR) groups, three weeks after arrival at the vivarium. Caloric restriction was introduced gradually over a period of 1 week until caloric intake reached 60% of that of the ad libitum intake, for each strain. This regimen was followed for an additional 6-week period and the mice in this study were euthanized at approximately 19 months of age. The CR group was fed NIH-31 diet formulated to provide an intake of essential nutrients equivalent to that of the AL group on a per mouse basis.
4.4. Preparation of tissue homogenate
Mice were euthanized by cervical dislocation and brains were removed and dissected in cold, into regions containing cortex/hippocampus, striatum, cerebellum and brainstem. Each region was immediately placed in ice-cold buffer containing 50 mmol/L potassium phosphate, 2 mmol/L EDTA, and 0.1 mmol/L butylated hydroxytoluene, pH 7.4, and stored at −80°C. Tissues were subsequently homogenized in 10 vol (w/v) of 10 mM Tris buffer containing 0.32 M sucrose and 1 mM EDTA, pH 7.1.
4.5. Sample preparation for glutathione analysis
Aliquots (300 μl) of the crude homogenate were mixed with 100 μl of ice-cold 20% (w/v) meta-phosphoric acid, incubated for 30 min in cold, and centrifuged for 20 min at 14,000 × g at 4°C. Supernatants were transferred to autosample vials and either injected immediately or stored at −80°C. For the measurement of protein-glutathione mixed disulfides (protein-SSG), the protein pellet from the initial precipitation of homogenates was resuspended and subjected to extensive washing to remove the free (nonprotein-bound) GSH and GSSG. Washing consisted of three cycles of resuspension of the precipitate in 1.5 ml of 5% (w/v) MPA containing 0.5 mmol/L EDTA, and a 15 s sonication on ice. After each wash, precipitated protein was recovered by centrifugation at 18,000 × g for 20 min at 4°C, and the supernatant was discarded. Protein-bound GSH was released by incubation of protein pellets in 100 mmol/L phosphate buffer, pH 7.4, containing 0.1 mM DTT for 1.5 h at 37°C. The protein content of the homogenates was determined by the BCA protein assay, according to the manufacturer's instructions (Pierce, Rockford, IL).
4.6. HPLC-coulometric EC detection
The procedures for the measurement of GSH and GSSG, described in detail previously (Rebrin et al., 2003), were designed to minimize artificial oxidation of GSH to GSSG. GSH and GSSG were separated by HPLC, equipped with a Shimadzu Class VP solvent delivery system (Shimadzu Corp., Kyoto, Japan), using a reverse-phase C18 Luna (II) column (3 μm; 4.6 × 150 mm), obtained from Phenomenex (Torrance, CA, USA). The mobile phase for isocratic elution consisted of 25 mmol/L monobasic sodium phosphate, 0.3 mmol/L of the ion-pairing agent 1-octane sulfonic acid, 1% (v/v) acetonitrile, pH 2.7, adjusted with 85% phosphoric acid. The flow rate was 0.7 ml/min. Under these conditions, the separation of aminothiols was completed in 35 min; GSSG was the last eluting peak, with a retention time of 30 min. Deproteinated samples were injected directly onto the column using a Shimadzu autosampler (Shimadzu Corp.). Calibration standards were prepared by the dilution of 2 mmol/L stock solutions of GSH or GSSG in 5% (w/v) meta-phosphoric acid, and injected at regular intervals to ensure uniform standardization. Each sample was injected twice, and the average of the peak areas was used for calculations of the aminothiol concentrations. Following HPLC separation, aminothiols were detected with a model 5600 CoulArray electrochemical detector (ESA, Inc., Chelmsford, MA) equipped with a four-channel analytical cell. Increasing potentials of +400, +600, +750, and +875 mV were applied on channels 1−4, respectively. GSH was detected on channel 3 (+750 mV) whereas GSSG was detected on channel 4 (+875 mV). The low potentials on channels 1 (+400 mV) and 2 (+600 mV) were used as an oxidative screen to eliminate interfering compounds that oxidize at a lower voltage.
4.7. Determination of redox potential
Potentials of the glutathione redox couple in homogenates were calculated by using experimentally determined concentrations of GSH and GSSG in the Nernst equation, as follows:
E0 is the standard potential of GSH (−270 mV at pH 7.4 in homogenate), R is the gas constant (8.31 J/deg mol), T is the absolute temperature (310 K), n is the number of electrons transferred (2) and F is the Faraday constant (96,406 J/V).
4.8. Statistical analysis of data
Data are presented as the mean ± SE for each brain region for separate groups of 5−8 mice of each strain. To determine the effects of age, mouse strain and brain regions on content of aminothiols, protein-SSG, and glutathione redox potential, data were analyzed by 3-way analyses of variance, with Age and Strain as between groups factors and Region as a within-group factor. For the effects of caloric restriction, the same measures were considered in 3-way analyses involving Strain, Diet, and Region as the factors. Planned individual comparisons between strain, age, and diet groups were performed using single degree-of-freedom F tests within the three-way interaction for each set of data.
Acknowledgements
This research was supported by grants R01 AG13563 and P01 AG022550 from the National Institutes of Health - National Institute on Aging.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Aoyama K, Suh SW, Hamby AM, Liu J, Chan WY, Chen Y, Swanson RA. Neuronal glutathione deficiency and age-dependent neurodegeneration in the EAAC1 deficient mouse. Nat. Neurosci. 2006;9:119–126. doi: 10.1038/nn1609. [DOI] [PubMed] [Google Scholar]
- Barja G. Free radicals and aging. Trends Neurosci. 2004;27:595–600. doi: 10.1016/j.tins.2004.07.005. [DOI] [PubMed] [Google Scholar]
- Chesselet MF, Delfs JM. Basal ganglia and movement disorders: an update. Trends Neurosci. 1996;19:417–422. doi: 10.1016/0166-2236(96)10052-7. [DOI] [PubMed] [Google Scholar]
- Collier TJ, Coleman PD. Divergence of biological and chronological aging: evidence from rodent studies. Neurobiol. Aging. 1991;12:685–693. doi: 10.1016/0197-4580(91)90122-z. [DOI] [PubMed] [Google Scholar]
- Cotgreave IA, Gerdes RG. Recent trends in glutathione biochemistry--glutathione-protein interactions: a molecular link between oxidative stress and cell proliferation? Biochem. Biophys. Res. Commun. 1998;242:1–9. doi: 10.1006/bbrc.1997.7812. [DOI] [PubMed] [Google Scholar]
- Droge W. Free radicals in the physiological control of cell function. Physiol. Rev. 2002;82:47–95. doi: 10.1152/physrev.00018.2001. [DOI] [PubMed] [Google Scholar]
- Dubey A, Forster MJ, Lal H, Sohal RS. Effect of age and caloric intake on protein oxidation in different brain regions and on behavioral functions of the mouse. Arch. Biochem. Biophys. 1996;333:189–197. doi: 10.1006/abbi.1996.0380. [DOI] [PubMed] [Google Scholar]
- Floyd RA, Hensley K. Oxidative stress in brain aging. Implications for therapeutics of neurodegenerative diseases. Neurobiol. Aging. 2002;23:795–807. doi: 10.1016/s0197-4580(02)00019-2. [DOI] [PubMed] [Google Scholar]
- Forster MJ, Dubey A, Dawson KM, Stutts WA, Lal H, Sohal RS. Age-related losses of cognitive function and motor skills in mice are associated with oxidative protein damage in the brain. Proc. Natl. Acad. Sci. USA. 1996;93:4765–4769. doi: 10.1073/pnas.93.10.4765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forster MJ, Lal H. Estimating age-related changes in psychomotor function: influence of practice and of level of caloric intake in different genotypes. Neurobiol. Aging. 1999;20:167–176. doi: 10.1016/s0197-4580(99)00041-x. [DOI] [PubMed] [Google Scholar]
- Forster MJ, Morris P, Sohal RS. Genotype and age influence the effect of caloric intake on mortality in mice. FASEB J. 2003;17:690–692. doi: 10.1096/fj.02-0533fje. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forster MJ, Sohal BH, Sohal RS. Reversible effects of long-term caloric restriction on protein oxidative damage. J. Gerontol. A. Biol. Sci. Med. Sci. 2000;55:B522–529. doi: 10.1093/gerona/55.11.b522. [DOI] [PubMed] [Google Scholar]
- Gage FH, Dunnett SB, Bjorklund A. Age-related impairments in spatial memory are independent of those in sensorimotor skills. Neurobiol. Aging. 1989;10:347–352. doi: 10.1016/0197-4580(89)90047-x. [DOI] [PubMed] [Google Scholar]
- Geinisman Y, Detoledo-Morrell L, Morrell F, Heller RE. Hippocampal markers of age-related memory dysfunction: behavioral, electrophysiological and morphological perspectives. Prog. Neurobiol. 1995;45:223–252. doi: 10.1016/0301-0082(94)00047-l. [DOI] [PubMed] [Google Scholar]
- Gerhard GS, Kaufmann EJ, Wang X, Erikson KM, Abraham J, Grundy M, Beard JL, Chorney MJ. Genetic differences in hepatic lipid peroxidation potential and iron levels in mice. Mech. Ageing Dev. 2002;123:167–176. doi: 10.1016/s0047-6374(01)00329-3. [DOI] [PubMed] [Google Scholar]
- Hof PR, Cox K, Morrison JH. Quantitative analysis of a vulnerable subset of pyramidal neurons in Alzheimer's disease: I. Superior frontal and inferior temporal cortex. J. Comp. Neurol. 1990;301:44–54. doi: 10.1002/cne.903010105. [DOI] [PubMed] [Google Scholar]
- Hyman BT, Van Hoesen GW, Damasio AR, Barnes CL. Alzheimer's disease: cell-specific pathology isolates the hippocampal formation. Science. 1984;225:1168–1170. doi: 10.1126/science.6474172. [DOI] [PubMed] [Google Scholar]
- Jones DP. Redox potential of GSH/GSSG couple: assay and biological significance. Methods Enzymol. 2002;348:93–112. doi: 10.1016/s0076-6879(02)48630-2. [DOI] [PubMed] [Google Scholar]
- Joseph JA, Roth GS. Altered striatal dopaminergic and cholinergic reciprocal inhibitory control and motor behavioral decrements in senescence. Ann. N. Y. Acad. Sci. 1988;521:110–122. doi: 10.1111/j.1749-6632.1988.tb35269.x. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Gage FH. Genetic determinants of adult hippocampal neurogenesis correlate with acquisition, but not probe trial performance, in the water maze task. Eur. J. Neurosci. 2002;16:129–136. doi: 10.1046/j.1460-9568.2002.02042.x. [DOI] [PubMed] [Google Scholar]
- Lee CK, Weindruch R, Prolla TA. Gene-expression profile of the ageing brain in mice. Nat. Genet. 2000;25:294–297. doi: 10.1038/77046. [DOI] [PubMed] [Google Scholar]
- Liu R-M. Down-regulation of gamma-glutamylcysteine synthetase regulatory subunit gene expression in rat brain tissue during aging. J. Neurosci. Res. 2002;68:344–351. doi: 10.1002/jnr.10217. [DOI] [PubMed] [Google Scholar]
- Liu R, Choi J. Age-associated decline in gamma-glutamylcysteine synthetase gene expression in rats. Free. Radic. Biol. Med. 2000;28:566–574. doi: 10.1016/s0891-5849(99)00269-5. [DOI] [PubMed] [Google Scholar]
- Markowska AL, Breckler SJ. Behavioral biomarkers of aging: illustration of a multivariate approach for detecting age-related behavioral changes. J. Gerontol. A. Biol. Sci. Med. Sci. 1999;54:B549–566. doi: 10.1093/gerona/54.12.b549. [DOI] [PubMed] [Google Scholar]
- Morrison JH, Hof PR. Life and death of neurons in the aging brain. Science. 1997;278:412–419. doi: 10.1126/science.278.5337.412. [DOI] [PubMed] [Google Scholar]
- Nicolle MM, Gonzalez J, Sugaya K, Baskerville KA, Bryan D, Lund K, Gallagher M, McKinney M. Signatures of hippocampal oxidative stress in aged spatial learning-imparied rodents. Neuroscience. 2001;107:415–431. doi: 10.1016/s0306-4522(01)00374-8. [DOI] [PubMed] [Google Scholar]
- Rebrin I, Bayne A-CV, Mockett RJ, Orr WC, Sohal RS. Free aminothiols, glutathione redox state and protein mixed disulphides in aging Drosophila melanogaster. Biochem. J. 2004;382:131–136. doi: 10.1042/BJ20040506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebrin I, Kamzalov S, Sohal RS. Effects of age and caloric restriction on glutathione redox state in mice. Free. Radic. Biol. Med. 2003;35:626–635. doi: 10.1016/s0891-5849(03)00388-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer FQ, Buettner GR. Redox environment of the cell as viewed through the redox state of the glutathione disulfide/glutathione couple. Free. Radic. Biol. Med. 2001;30:1191–1212. doi: 10.1016/s0891-5849(01)00480-4. [DOI] [PubMed] [Google Scholar]
- Serrano F, Klann E. Reactive oxygen species and synaptic plasticity in the aging hippocampus. Ageing Res. Rev. 2004;3:431–443. doi: 10.1016/j.arr.2004.05.002. [DOI] [PubMed] [Google Scholar]
- Sohal RS, Ku H-H, Agarwal S, Forster MJ, Lal H. Oxidative damage, mitochondrial oxidant generation and antioxidant defenses during aging and in response to food restriction in the mouse. Mech. Ageing Dev. 1994;74:121–133. doi: 10.1016/0047-6374(94)90104-x. [DOI] [PubMed] [Google Scholar]
- Sohal RS, Weindruch R. Oxidative stress, caloric restriction, and aging. Science. 1996;273:59–63. doi: 10.1126/science.273.5271.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spindler SR. Rapid and reversible induction of the longevity, anticancer and genomic effects of caloric restriction. Mech. Ageing Dev. 2005;126:960–966. doi: 10.1016/j.mad.2005.03.016. [DOI] [PubMed] [Google Scholar]
- Suh JH, Wang H, Liu R-M, Liu JK, Hagen TM. (R)-alpha-Lipoic acid reverses the age-related loss in GSH redox status in post-mitotic tissues: evidence for increased cysteine requirement for zGSH synthesis. Arch. Biochem. Biophys. 2004;423:126–135. doi: 10.1016/j.abb.2003.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uecker A, Gonzalez-Lima F, Cada A, Reiman EM. Behavior and brain uptake of fluorodeoxyglucose in mature and aged C57BL/6 mice. Neurobiol. Aging. 2000;21:705–718. doi: 10.1016/s0197-4580(00)00099-3. [DOI] [PubMed] [Google Scholar]
- Upchurch M, Wehner J. DBA/2Ibg mice are incapable of cholinergically-based learning in the morris water task. Pharmacol. Biochem. Behav. 1988;29:325–329. doi: 10.1016/0091-3057(88)90164-5. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Gur RC, Wang GJ, Fowler JS, Moberg PJ, Ding YS, Hitzemann R, Smith G, Logan J. Association between decline in brain dopamine activity with age and cognitive and motor impairment in healthy individuals. Am. J. Psychiatry. 1998;155:344–349. doi: 10.1176/ajp.155.3.344. [DOI] [PubMed] [Google Scholar]
- Wang H, Liu H, Liu RM. Gender difference in glutathione metabolism during aging in mice. Exp Gerontol. 2003;38:507–517. doi: 10.1016/s0531-5565(03)00036-6. [DOI] [PubMed] [Google Scholar]
- Wardman P. Reactions of thiol radicals. In: Packer L, Cadenas E, editors. Biothiols in Health and Disease. Marcel Decker; New York: 1995. pp. 1–19. [Google Scholar]
- Winterbourn CC. Concerted antioxidant activity of glutathione and superoxide dismutase. In: Packer L, Cadenas E, editors. Biothiols in Health and Disease. Marcel Decker; New York: 1995. pp. 117–134. [Google Scholar]