Abstract
Rb/E2F regulates many genes that encode proteins required for the cell cycle. Using affymetrix microarrays we previously identified genes regulated by the Rb proteins p130 and p107, many of which are involved in the cell cycle. Several genes with unknown functions were also repressed by p130 and p107, of which some have recently been found to have various roles in mitosis, the spindle checkpoint and cytokinesis. This study focuses on the regulation of borealin/dasra/cdca8, which encodes a recently discovered member of the chromosomal passenger complex. It is recorded that borealin is a cell cycle regulator, down-regulated in response to p53/Rb-signaling, and up-regulated in many types of cancerous tissues.
Keywords: mitosis, cell cycle, Rb, E2F, p53
1. Introduction
Progression through the cell cycle culminates at mitosis, in the division into two daughter cells. Entry into mitosis in diverse organisms is a control point for a number of checkpoint pathways that ensure that previous events have been properly carried out (Elledge, 1996; Molinari, 2000). For example, DNA damage blocks entry into mitosis leaving cells in G2 until the damage is repaired (O’Connell et al., 2000). In mammalian cells, rapid G2 arrest is mediated by the ATM/ATR-dependent inactivation of CDK1, the cyclin-dependent kinase (CDK) that drives cells into mitosis (O’Connell et al., 2000). Long-term arrest in G2 is ensured by pathways activated by the p53 tumour suppressor, which also plays important roles in controlling progression from G1 into S phase, and through S phase (Agarwal et al., 1998; Taylor and Stark, 2001; Vogelstein et al., 2000). p53 is frequently inactivated in human tumours and suppresses tumourigenesis by inducing cell cycle arrest or cell death when damage is detected. The biological effects of p53 are generally mediated by its ability to bind to specific promoter elements and activate the transcription of a diverse array of genes (Vogelstein et al., 2000).
An important pathway triggered by p53 results in the down-regulation of a large number of genes that encode proteins needed to enter into and progress through mitosis (Badie et al., 2000; Jackson et al., 2005). p21/waf1, a transcriptional target of p53, encodes a protein that inhibits multiple CDKs leading to the dephosphorylation of Rb family proteins (Boulaire et al., 2000). In their poorly phosphorylated form, Rb proteins bind to members of the E2F family, which bind to specific promoter elements (Harbour and Dean, 2000). Rb/E2F complexes are potent repressors of transcription and mediate the down-regulation of genes needed for G2 and M in response to p53 signaling (Harbour and Dean, 2000; Jackson et al., 2005; Taylor and Stark, 2001). When not bound to Rb, E2F proteins can activate the transcription of many of the same genes that are repressed by the Rb/E2F complex. Rb/E2F target genes are important for both the G1/S and G2/M cell cycle transitions (Harbour and Dean, 2000).
Once a cell has entered mitosis and all chromosomes have attached to the mitotic spindle and has aligned at the metaphase plate the cell enters anaphase, during which sister chromatids are segregated to opposite poles of the cell. Shortly after anaphase, the cleavage furrow forms along the plane defined by the metaphase plate. Many proteins are found to localise to the spindle midzone and cleavage furrow where they carry out diverse roles to bring about cell division (Guertin et al., 2002). Previously it was found that rb6k, prc1, plk1, ect2, anilin, and survivin, all of which encode proteins found at the spindle midzone and cleavage furrow are repressed when DNA is damaged, but only in cells with intact Rb family proteins (Jackson et al., 2005). We report on the expression of borealin, also known as cdca8 and Dasra B, a member of the chromosomal passenger complex, which travels to the spindle midzone at anaphase along with Survivin, Incenp and Aurora B (Gassmann et al., 2004; Sampath et al., 2004). We show that borealin is regulated in a cell cycle dependent manner, characteristic of Rb/E2F targets and that Borealin expression is elevated in glioblastoma, lymphoma and colon cancer. We also note that borealin is repressed by high levels of p53, but basal levels of borealin are not solely controlled by p53 status in cancer-derived cell lines.
2. Materials and methods
2.1 Cell lines and culture conditions
Cells were grown in a humidified atmosphere containing 10% CO2 in Dulbecco’s minimal essential medium (Gibco). All cell lines were monitored for mycoplasma infection by staining with Hoechst 33342. Any infected cell lines were treated with mycoplasma removal agent (ICN) for 7 d and the absence of infection was confirmed by staining with Hoechst 33342. Only cells lacking infection were used in experiments. Mammary epithelial growth medium (Cambrex biosciences), F12K medium, or RPMI (HyClone) (supplemented with antibiotics and 10% fetal bovine serum) (Gibco) depending on the cell line. Transfections were carried out using GenePorter (Gene Therapy Systems) Lipofectamine 2000 (Invitrogen) and Fugene 6 (Roche) with similar results. NARF2 cells were derived from U20S osteosarcoma cells that contain wild-type p53 and hDM2, by transfection with a construct that encodes an IPTG-inducible p14ARF (Stott et al., 1998). A number of derivatives of NARF2 were created by first infecting NARF2 cells with a retrovirus containing pBabe-Bleo-Eco vector. Infected cells were pooled and then cells were individually infected with retroviruses with the following vectors: pLXSN, pLXSN-GSE56, pBabe-Puro, pBabe-puro-Cyclin D1/Cdk fusion or were infected with lentiviral vectors encoding hDM2 cDNA or short hairpin RNA targeting p53 (Jackson et al., 2005). Other cell lines were obtained from investigators in the Department of Biological Sciences at the University of Toledo. These cell lines were originally obtained form the American Type Tissue Collection. Multiple frozen stocks were prepared and cell lines carried in culture for a maximum of two months before being discarded and replaced with a fresh vial. Chemicals were obtained from Sigma-Aldrich unless otherwise noted. Thymidine was used at a concentration of 0.2 mM, nocodazole at 200 ng/ml, adriamycin at 0.2 mg/ml and IPTG at 100mM. Double thymidine block was carried out as previously described (Whitfield et al., 2000). Briefly, Hela cells were blocked in S phase by exposure to 0.2 mM thymidine for 18 h. Thymidine was removed, cells were incubated for 9 h and thymidine was re-added. Eighteen hours later, thymidine was removed to allow synchronous progression of cells through the cell cycle.
2.2 Western blotting
Whole cell extracts were prepared by incubating cell pellets in lysis buffer containing 50 mM Tris (pH 8.0), 150 mM NaCl, 1.0% NP-40, 10 μg/ml aprotinin, 1mM phenylmethane sulfonyl fluoride, 5 μg/ml leupeptin, 5 μg/ml pepstatin and 1 mM NaVO3 and 1mM NaF. Extracts containing equal quantities of proteins, determined by the Bradford method were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (12.5% acrylamide at a ratio of 37.5:1 acrylamide:bisacrylamide) and transferred to polyvinylidene difluoride membranes (Millipore, Bedford, MA).
Immunoblots were blocked with phosphate buffered saline containing 5% nonfat dry milk. Antibodies to the Flag-tag that were directly conjugated to horse-radish peroxidase were obtained from Bethyl Laboratories and used at a dilution of 1:1000. Antibodies to p53 directly conjugated to horse-radish peroxidase (Santa Cruz Biotechnology) and antibodies to Actin (NeoMarkers) were used at a dilution of 1:2000. Goat anti-mouse secondary antibodies conjugated to horse-radish peroxidase (1:2000) were obtained from Santa Cruz Biotechnologies. Bound antibodies were detected using enhanced chemiluminescence (Dupont, Wilmington, DE)(Clifford et al., 2003).
2.3 Reverse transcription-polymerase chain reaction (RT-PCR)
RNA was isolated using Trizol (Invitrogen) according to the manufacturer’s instructions and RT-PCR was carried out using enzymes from Promega and according to their instructions.
When comparing levels of mRNA by RT-PCR, the total RNA content of starting samples was first determined by measuring the absorbance at 260 nm (as an indication of the amount of 18S and 28S RNAs which constitute the majority RNA in the sample). Samples were then added into the RT-PCR reaction in such a manner as to equalise RNA input based on the absorbance reading. Under these conditions, we observed that gapdh did not vary substantially suggesting that it serves as an appropriate internal loading control for the cell lines we have analysed (see Fig. 7). To construct the C-terminal Flag-tagged Borealin protein, the tag was incorporated into the reverse PCR primer, and PCR products were cloned into the pcDNA 3.2 expression vector using topo cloning (Invitrogen). Two distinct mRNAs for borealin were isolated, 1 in which the first intron was spliced (same as accession number: NM_018101) and another containing the first intron (same as accession number: BC001651). RT-PCR was used to detect the expression of borealin that contains intron 1 using a forward primer that binds within intron 1 (5′ CGTCCCT ACCCAGTTTCTTG 3′). To detect expression of unspliced borealin, a forward primer was designed that was complementary to a sequence within exon 1 (5′ TTTTGCTCAGCCC TTGTCTC 3′). The reverse primer was the same for both sets of reactions (5′ GGATGGAGGACAC CTTTT GA 3′). PCR reactions were prepared by using JumpStart Taq ReadyMix according to manufacturer’s instructions (Sigma-Aldrich). DNA was amplified for 30 cycles by denaturing at 95°C for 1 m, annealing for 1 m at 57°C, and an extension period of 5 m at 72°C. To control for loading, gapdh cDNA was also amplified (forward primer 5′AAATCCCATCACCA TCTTCC 3′) (reverse primer 5′ GTCCACCACCCTGTTGCTGC 3′). PCR products from reactions containing the primer within intron 1 and gapdh were separated using agarose gels. PCR products amplified with the primer complimentary to exon 1 were separated on an acrylamide gel (0.5 X TBE, 5% acrylamide) and stained with ethidium bromide.
Figure 7. Splice variants of borealin.
The expression of two major splice variants of borealin in a number of different cell lines was surveyed using RT-PCR. BorealinL represents the long splice variant containing intron 1 and borealinS represents the short splice variant lacking the first intron. (A) Schematic of borealin splice variants. Forward primers were designed that annealed either to a region of exon 1 (primer “A”) or to intron 1 (primer “B”). A reverse primer anneals to a sequence in exon 7. The combination of primer A and the reverse primer allows the detection of both splice variants. The combination of primer B and the reverse primer detects only the borealinL variant. (B) RT-PCR reaction to detect both borealinL and borealinS variants. DNA was separated by polyacrylamide gel electrophoresis to resolve the two bands. Positive controls were generated by using cloned cDNAs representing both splice variants as the template in the PCR reaction. Reactions contained the primers A and REV. (C) RT-PCR reaction to detect the borealinL variant. A typical reaction using primer B and the reverse primer is shown. To assess loading, RT-PCR was carried out using primers specific for gapdh. (D) Expression of Borealin protein from borealin splice variants. HEK 293 cells were transiently transfected with cDNA that contained intron 1 (borealinL) or cDNA without intron one (borealinS). Cells were treated with nocodazole for 16 h to arrest them in mitosis. UT: untreated.
2.4 Northern blotting
Total RNA was extracted with Trizol reagent (Invitrogen) according to manufacturer’s instructions, separated by electrophoresis in a 1% denaturing agarose gel, transferred to Hybond N+ nylon membranes (Amersham Biosciences) and probed with 32P-labeled DNA probes.
2.5 FACS Analysis
Cells harvested with trypsin were washed with phosphate buffered saline and fixed by dropping them into 1 ml of 100% ethanol pre-chilled to −20°C. To stain the cells for DNA content, 0.5 mg/ml propidium iodide along with 5 μg/ml RNaseA was added to the cell solution and incubated for 30 m. Cells were then analysed by flow cytometry using CellQuest software. Cylchred software was used in analysing the percentage of cells in each phase of the cell cycle. Ten thousand cells were analysed for each sample.
2.6 Analysis of Affymetrix and expressed sequence tag (EST) data
Targets of Rb-dependent repression were identified using affymetrix microarrays (Jackson et al., 2005). mRNA was isolated from MEFs exposed to adriamycin (345nM) for either 12 or 24 h. Wild-type MEFs were compared to MEFs lacking p130 and p107, members of the Rb family, in order to identify genes repressed preferentially in the wild-type cells. Genes repressed in a p130/p107-dependent manner were identified as described (Jackson et al., 2005). Data for some genes were not previously published due to a scarcity of information regarding their functional significance. Data for those genes are now shown in Table 1. EST frequencies generated from normal human and tumour tissues were downloaded from the Cancer Genome Anatomy Program (CGAP) web page (http://cgap.nci.nih.gov). Several tissues were removed from the analysis due to a lack of libraries. Frequencies were uploaded to the EPCLUST web page for hierarchical clustering (http://ep.ebi.ac.uk/EP/EPCLUST).
Table 1.
Genes downregulated by DNA damage in a p130/p107-dependent manner.
| 12h
|
24h
|
|||||
|---|---|---|---|---|---|---|
| Name | Function | Accession Number | KO | WT | KO | WT |
| cdca8/borealin/dasra B | mitosis/cytokinesis | AW122331 | −1.6 | −8 | −2 | −9 |
| racgap1/mgcracgap | cytokinesis | AW122347 | −2.1 | −12 | −2.5 | −16 |
| diaph3 | actin cytoskeleton | AF094519 | −1.2 | −4.3 | −1.7 | −4.7 |
| aknt/nusap1 | mitotic spindle | AA275196 | −1.4 | −5.6 | −3.1 | −13 |
| Esk | similar to mps1 (spindle checkpoint) | M86377 | 1.2 | −5.6 | −1.4 | −5.4 |
| Hurp | kinetochore | AI510131 | 1 | −8.2 | −1.3 | −10 |
| brrn1/barren/hcap-h | chromosome condensation | AW213883 | −1.3 | −8.6 | −1.7 | −9.4 |
| fin16/smc2l1 | chromosome condensation | U42385 | −1.1 | −5.7 | −1.2 | −9.3 |
| Sororin | chromatid cohesion | AI877184 | −1.1 | −2.4 | −1.4 | −3.1 |
| p15(paf) | UV response | AI122538 | −1.6 | −4.1 | −4.3 | −11 |
| rad51ap1 | DNA damage response | U93583 | −1.7 | −4.4 | −1.8 | −5.4 |
| ubp1 | protein degradation | AI848382 | −1.8 | −4 | −1.9 | −4.9 |
| Dek | transcription regulator | AA733594 | −1.3 | −4.2 | −1.5 | −4 |
| Solt | transcription regulator | AA896295 | −1.4 | −4.6 | −1.3 | −3.2 |
| ledgf/p75 | transcription regulator | AW047331 | 1.1 | −2 | 1.1 | −2.2 |
| Ctcf | regulator of myc | U51037 | −1 | −5.8 | 1.1 | −2.1 |
| hat1 | histone modification | AW125218 | −1.2 | −7.9 | −1.4 | −7.8 |
| hmg2 | chromatin structure | X67668 | −2.1 | −7.4 | −5.7 | −17 |
| u50 snoRNA | RNA metabolism | AW045664 | −1.2 | −5.4 | 1 | −2.2 |
| lsm3 | RNA metabolism | AA795062 | −1.1 | −2.7 | 1.1 | −2.8 |
| kiaa1574/fam29a | unknown | AI197337 | 1.2 | −3.2 | −1 | −3.4 |
| Hfleg | unknown | AI851770 | −1.3 | −3.2 | −1.3 | −7.7 |
| c4orf15 | unknown | AI462312 | −1.1 | −2.5 | −1 | −2.2 |
| p90 autoantigen | unknown | AA590345 | 1.1 | −6.5 | −1.1 | −8.2 |
Wild-type (WT) or p130/p107-null MEFs (KO) were exposed to adriamycin (ADR; 345nM) for either 12 and 24 hours. Fold repression determined with Affymetrix microarrays is shown. Numbers in italics are estimates due to undetectable expression in treated samples).
2.7 Analysis of human tissues by immunohistochemistry
Human tissue arrays (TARP3) were provided by the Tissue Array Research Program of the National Cancer Institute. Each array contains approximately 500 tissues cores (0.6mm diameter). Immunohistochemistry was carried out using a rabbit antisera prepared against a Borealin-glutathione S-transferase fusion proteins purified from E. coli. Non-specific antibodies were removed by binding to glutathione-S-transferase immobilized on glutathione-sepharose. Specificity of purified antibodies was confirmed by immunfluorescence. Immunohistochemistry was carried out using the ABC kit from Vector laboratories according to the manufacturer’s instructions. Antigen retrieval was carried out by boiling slides for 15 m in a rice cooker in the presence of 10 mM Sodium Citrate (pH 6.0).
3. Results and Discussion
3.1 Cell cycle regulation of borealin
Affymetrix microarrays were used to find genes down-regulated in response to DNA damage caused by adriamycin. Of those that require the Rb family proteins p130 and p107 for repression, many were known to play a role in the cell cycle (Jackson et al., 2005). Specifically, we observed that 24 genes encoding proteins required for mitosis, 3 for the spindle checkpoint and 10 for DNA synthesis required p130 and p107 to be efficiently down-regulated in response to DNA damage (Jackson et al., 2005). Twenty four genes that were similarly down-regulated were categorised as having unknown or miscellaneous functions (Jackson et al., 2005). More recent genome annotation has indicated that many of those 24 genes also play a role in the cell cycle. For example, hurp has recently been found to play an important role in spindle formation and kinetochore capture (Koffa et al., 2006; Wong and Fang, 2006). We found that it was repressed 8 to 10 fold in wild-type cells exposed to adriamycin, but only 1–1.3 fold in p130/p107-null cells (Table 1). Sororin encodes a protein that was recently implicated in chromatid cohesion (Rankin et al., 2005). The affymetrix experiment also suggests that it is a target of Rb-dependent repression when DNA is damaged. Of particular interest was the expression of borealin, a new member of the chromosomal passenger complex (Gassmann et al., 2004; Sampath et al., 2004). The repression of borealin in wild-type cells after adriamycin treatment was much more substantial than that observed in p130/p107-null cells, although the knockout cells did show some repression (Table 1). The results suggest that the transcription of borealin is under the control of the Rb family. To confirm the effect of adriamycin, the expression of borealin was examined in human cells that contain wild type p53. HCT116 p53 +/+. Cells were treated with adriamycin for 24, 48, and 72 hs and RNA was collected for Northern blotting to measure borealin levels. The expression of borealin was undetectable after 24 h of treatment and remained undetectable throughout the course (Fig. 1A). Therefore, DNA damage leads to decreased expression of borealin in HCT116 cancer cells.
Figure 1. The effect of DNA damage and p14ARF on the expression of borealin.
borealin mRNA levels were determined by the Northern method. To control for loading, the membranes were stripped and probed for gapdh. Levels of borealin protein were examined using the Western method. (A) Effect of DNA damage on borealin expression. HCT 116 cells containing wild-type p53 were treated with adriamycin to damage DNA for 24, 48, and 72 h. (B) Effect of p14ARF on borealin expression. NARF2 cells, which contain an IPTG-inducible p14ARF protein were treated with IPTG for 24, or 72 h. Induction of p14ARF blocks the ability of hDM2 to degrade p53 leading to an increase in the level of p53 protein. The effect on the levels of borealin is shown. (C) Effect of Adriamycin on the level of Borealin protein. HCT116 with wild-type p53 were treated with adriamycin (ADR; 0.2mg/ml) for the indicated times and analysed by western blotting with antibodies to either Borealin or p53. β actin was used as loading control. (D) Effect of Cisplatin on the level of Borealin. HCT116 cells were treated with cisplatin (1μg/ml) for the times indicated and analyzed as described in “C”. (E) Effect of DNA damage on Borealin protein levels in HT1080 cells. HT1080 fibrosarcoma cells with wild type p53 were treated with adriamycin (0.2mg/ml) or cisplatin (1μg/ml) and analysed by western blotting to determine levels of Borealin, p53 and β actin.
The down-regulation of borealin in HCT116 cells exposed to adriamycin may be due to high levels of p53 protein induced by DNA damage. To test this idea, NARF2 cells, with an IPTG inducible p14ARF were treated with IPTG for 24, 48, and 72 h. The addition of IPTG causes an increase in p14ARF protein, which in turn causes p53 to accumulate by blocking its degradation by hDM2. High levels of p53 in NARF2 cells induce cell cycle arrest (Stott et al., 1998). We observed that after 24 h of treatment with IPTG, borealin mRNA was expressed at a very low level and remained so through 72 h (Fig. 1B).
In order to determine if the effects of p53 and DNA damage on borealin mRNA resulted in a reduction in the amount of the encoded protein we raised a rabbit antiserum against a Borealin-glutathione-S transferase fusion protein. Western blotting detected the presence of a band of the appropriate size in HCT116 p53 +/+ that was down-regulated when the cells were exposed to adriamycin (Fig. 1C)(Kaur et al., 2007). Exposure of HCT116 p53 +/+ cells to cisplatin also resulted in the down-regulation of the level of Borealin protein, however not to the extent observed in response to adriamycin (Fig. 1D). Interestingly, the opposite trend was observed in HT1080 cells which also contain wild-type p53. Borealin was down-regulated in response to exposure to either adriamycin or cisplatin, however cisplatin was much more efficient at inducing this effect (Fig. 1E). Therefore, different DNA damaging agents have different abilities to down-regulate Borealin expression, and these differences are cell-type dependent.
Many genes that encode proteins involved in mitosis are regulated by Rb/E2F and are transcriptionally up-regulated in G2/M. When we analyzed borealin expression in Hela cells synchronized by a double thymidine block, we found low levels of borealin mRNA at zero hours post release, which represents the G1/S boundary (Fig. 2A and B). Steady state levels of borealin continued to increase as cells approached G2/M (Fig. 2A and B). Visual examination indicated that many rounded mitotic cells were evident at 10 h indicating that at this time point many cells are at the transition from G2 to M (our unpublished data). Maximal borealin expression was found at 12 h post release when cells started to exit mitosis. Borealin expression was reduced by 14 h post release, at time at which most cells have exited mitosis and entered G1 (Figs 2A and B).
Figure 2. Cell-cycle regulation of borealin.
Borealin expression was measured using the Northern method. To control for loading, membranes were stripped and probed for gapdh. (A) FACS analysis of cell cycle position after S phase synchronisation. Hela cells were synchronised by a double thymidine block and released to progress through the cell cycle. Cell cycle positions were determined by FACS analysis of propidium iodide stained nuclei and representative histograms are shown. (B) Cell cycle regulated expression of borealin mRNA. Total RNA was isolated from Hela cells synchronised using the double-thymidine block. Average borealin mRNA levels from two independent experiments corrected for loading are plotted. Cell cycle distribution determined by FACS as described in (A) is also plotted for comparison.
Borealin forms a complex with Incenp, Survivin, and Aurora B (Gassmann et al., 2004; Sampath et al., 2004). All four proteins localise to the inner centro-mere during prophase and metaphase, and then travel to the spindle mid-zone during anaphase (Vagnarelli and Earnshaw, 2004). Borealin can bind to DNA raising the possibility that it mediates attachment of the complex to the inner centro-mere (Klein et al., 2006). One of the roles of the chromosomal passenger complex appears to be to ensure that Aurora B kinase is properly located to phosphorylate its target proteins at the centromere and the spindle midzone (Vagnarelli and Earnshaw, 2004). The pattern of borealin expression we have observed could reflect a need for high levels of borealin protein during mitosis and through cytokinesis. Consistent with our observations with synchronized cells, the level of borealin mRNA was higher in HCT116 cells blocked in M with nocodazole (Fig. 3A). Cells blocked in S phase with high levels of thymidine had similar levels of borealin as asynchronously growing cells. Our observations on the pattern of borealin mRNA expression during the cell cycle are consistent with a recent study on the level of borealin protein. In that study, cells were synchronized by treating with nocodazole to block them in mitosis (Chang et al., 2006). Cells released from the block first showed an increase in borealin expression followed by a decrease. The decrease occurred ~3 h after most Cyclin B1 was degraded and ~3 h after many (~40%) of the cells had entered G1 (Chang et al., 2006). Together, these results indicate that the rise of borealin protein at mitosis is primarily due to transcriptional up-regulation.
Figure 3. Survey of borealin expression in cancer cell lines and tumour tissues.
mRNA levels were assessed using the Northern method (A, B and C) or estimated based on EST frequencies (D). (A) Effect of cell cycle arrest and p53 status on borealin mRNA levels. HCT 116 p53 +/+ and HCT 116 p53−/− cells were treated for 16 h with thymidine to block them in S phase or nocodazole to block them in mitosis. (B) Expression of borealin mRNA in asynchronously growing HT1080 LX and GSE cells. (C) Survey of borealin expression in human cancer cell lines. The p53 status of each cell line is shown. (Left Panel) Leukemia cell lines are shown in the left panel, prostate cancer cell lines in the right panel, and various additional cell lines are shown in the middle panel. A bar graph of the level of borealin corrected for loading (bor/gap) is shown. (D) EST representation of borealin in human cancer. The CGAP was searched to estimate the level of expression of borealin as well as birc5 (survivin), plk1, and ki67 in various cancerous tissues. EST frequencies were compiled and analysed by hierarchical cluster analysis. Red bars indicate a high level of expression of the gene, whereas black bars represent low levels of expression.
3.2 Basal levels of borealin in cancer cell lines
We observed that a derivative of HCT116 cells lacking p53 expressed higher basal levels of borealin compared to HCT116 with p53 (Fig. 3A). Similarly, when p53 was inactivated by expressing the dominant-negative fragment GSE56 in HT1080 cells that have wild-type p53, the basal level of borealin mRNA was elevated (Fig. 3B). The effect of knocking out the p53 gene in HCT116 was more pronounced than using the dominant-negative fragment in HT1080. Together, these data indicated that in isogenic systems, p53 controls the basal level of expression of borealin. We tested if this correlation was also evident in a number of tumour derived human cell lines that differ in p53 status. Asynchronously growing cells were used to collect RNA and the steady state level of borealin mRNA was detected using the Northern method. Borealin was easily detectable in all cancerous derived cells (Fig. 3C). The p53 status of each cell line was surveyed to determine if p53 function had an effect on expression of borealin. Unlike the case, in isogenic systems, we observed that the level of borealin did not correlate with p53 status in the tumour-derived cell lines analysed. For example in the p53 +/+ cell line, Jurkat, borealin expression levels were similar to the p53 null cell line K562. The Daudi (p53+/+) and TF-1 (hemizygous for p53, p53−/+) cell lines express similar levels of borealin mRNA while BV173 (p53+/+) had the highest level of borealin when loading was considered (Fig. 3C). HT1080, HCT 116 p53 +/+, and MCF7 all have wild type p53 and the level of borealin mRNA was easily detectable and slightly higher than in Hela cells in which p53 is inactivated by the E6 protein of human papilloma virus (Fig. 3C). In the mutant p53 cell line Du145, a similar level of borealin as compared to the p53 wild type cell line LNCaP and the null p53 cell line PC-3 (Fig. 3C). Therefore, although p53 contributes to the basal level of borealin expression, other factors must also control the basal levels of borealin. These additional factors appear to predominate when comparing multiple non-isogenic cancer cell lines and are likely to represent a complicated mixture of cell-cycle control proteins and transcription factors.
3.3 Expression of borealin in cancer
Products of a number of genes that are repressed by Rb-dependent pathways, such as PLK1 and KI67, are highly expressed in various cancers suggesting that they may be useful prognostic factors (Brown and Gatter, 2002; Steeg and Zhou, 1998; Wolf et al., 2000). To determine if the expression of borealin is associated with neoplastic transformation of human tissues, we queried CGAP to assess EST frequencies as a measure of borealin expression (see Materials and Methods). The well-known proliferation markers birc5 (survivin), plk1, and ki67 were analysed in parallel (Andersen and thor, 2002). These three genes, as well as borealin are highly over-represented in tumour tissue from various origins (Fig. 3D). Specifically, ESTs for borealin were more abundant in 24 of the different cancer tissues surveyed. The exceptions were cancers in the following categories: testis, lymphoreticular, head and neck, bone marrow and peripheral nervous system. It is not known why cancers from these tissues do not express more borealin than the corresponding normal tissue.
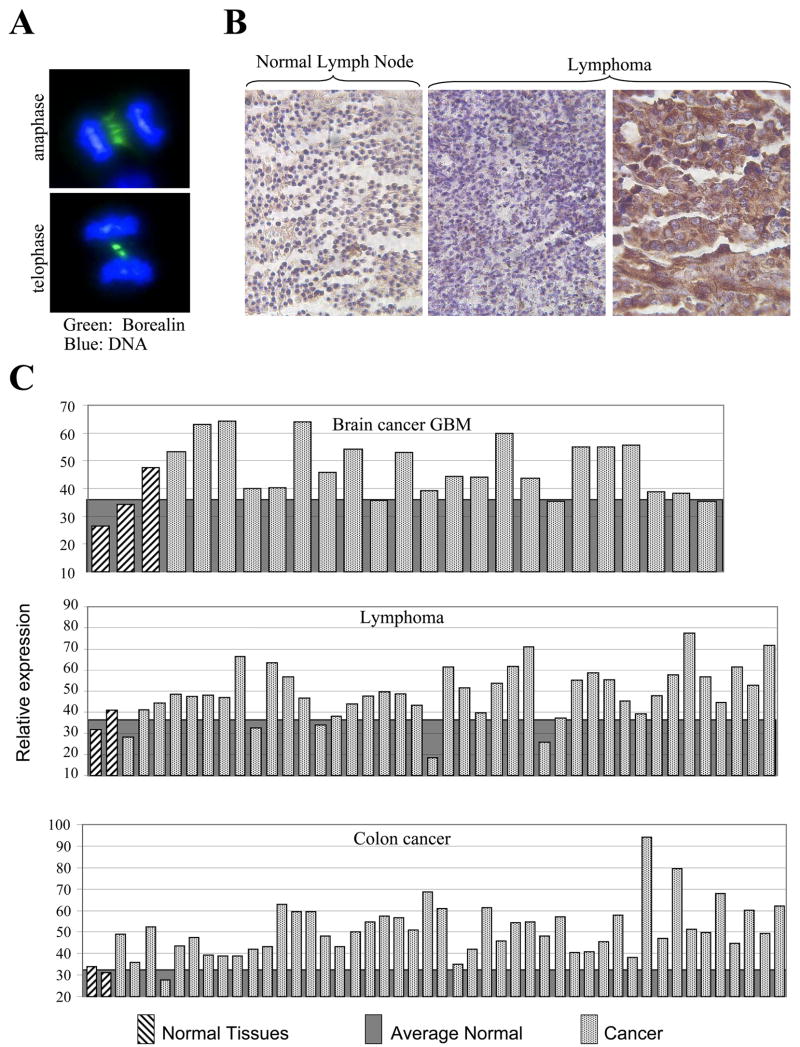
In a previous study, immunohistochemistry was used to analyse the level of Borealin protein in gastric cancer (Chang et al., 2006). Borealin was over-expressed in gastric cancer tissue and high levels of the protein correlated inversely with survival status (Chang et al., 2006). To extend these studies, we used immunohistochemistry with our polyclonal antiserum to compare the levels of Borealin in lymphoma, colon cancer and brain cancer. In order to test the specificity of our antibody we performed immunofluorescence on Hela cells and found that, as previously described, borealin was localised to the spindle mid-zone during anaphase and to the mid-body at telo-phase (Fig. 4A). Immunohistochemistry indicated that borealin staining in tumours was variable, with some tumours showing low levels of staining similar to normal tissues, while in other tumours borealin was elevated (for example see Fig. 4B). We observed that 45% of brain cancers expressed at least 50% higher levels of borealin compared to normal tissues. 34% of lymphomas and 61% of colon cancers expressed at least 50% higher levels of borealin compared to the corresponding normal tissues (Fig. 4C). Overall, our results agree with the immunohistochemistry analysis of borealin in gastric cancer and show that borealin is over-expressed in some, but not all lymphomas, brain cancers and colon cancers.
Figure 4. Comparison of Borealin levels in normal and cancer tissues.
Immunohistochemistry was performed on TARP3 tissue arrays containing approximately 500 paraffin embedded tissues. Each tissue was photographed with a digital camera and pixel intensities determined using the ImageJ program. Background levels captured from a region of the slide without tissue were subtracted from each normal and cancer value. In each graph, the average level of staining in the corresponding normal tissues is shown by the gray area.
3.4 Regulation of borealin by p53 signaling
Our results with NARF2 cells suggest that high levels of p53 can trigger down-regulation of borealin. However, p53 in NARF2 cells is induced indirectly by p14ARF which inactivates hDM2. We have previously confirmed that both p14ARF and p53 are induced in NARF2 cells and in the NARF2 derivatives used below (Jackson et al., 2005). hDM2 has been found to bind to Rb proteins and p14ARF shown to target myc-dependent transcription and ribosomal RNA synthesis (Cleveland and Sherr, 2004; Datta et al., 2004; Itahana et al., 2003; Qi et al., 2004). To test if p53 was critical for the down-regulation of borealin in NARF2 cells we inactivated it using several approaches. Derivatives of NARF2 cells infected with a retrovirus that directs the synthesis of a short hairpin RNA targeting p53 did not repress borealin when IPTG was added to up-regulate p14ARF (Fig. 5A). Expression of hDM2 or GSE56, a dominant-negative fragment of p53, abrogated the repression of borealin (Fig. 5A). These results show that p53 is critical for the repression of borealin that occurs in response to high levels of p14ARF.
Figure 5. Expression of borealin in derivatives of NARF2 cells.
NARF2 cells expressing the indicated proteins were treated with IPTG for 48 h. (A) Expression of borealin and p21/waf1 in NARF2 derivatives. mRNA levels for borealin and p21/waf1 were determined using the Northern method. Transferred membranes were also assessed for loading using gapdh. (B) Expression of p53 in NARF2 derived cell lines. Western blotting was used to determine the levels of p53 in each NARF2-derived cell line. β-Actin served as a loading control.
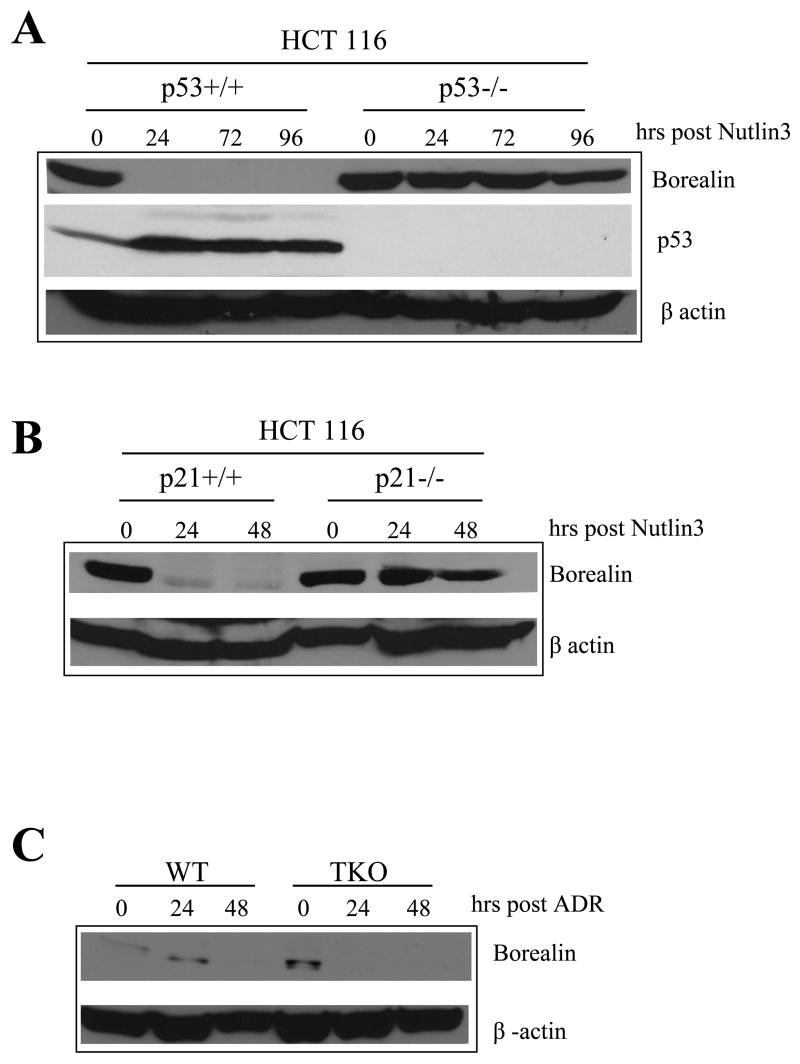
p53 can cause repression of cell cycle genes by inducing p21/WAF1 which inhibits CDKs. To test if CDKs are important in the effect of p53 on borealin, we over-expressed a cyclin D1-CDK2 fusion protein which mimics a CDK complex found at high levels in breast cancer (Chytil et al., 2004). The D1-CDK2 fusion has been shown to be constitutively active and capable of phosphorylating p105Rb (Chytil et al., 2004). Repression of borealin was still observed in the NARF2 derivatives expressing D1-CDK2, however repression was clearly defective when compared to control cells infected with the empty vector (pBpuro)(Fig. 5A). Northern blot analysis of p21/waf1 showed that it was no longer efficiently induced in cells containing shp53, hDM2, or GSE56 confirming that p53 function was defective in these cell lines (Fig. 5A). Western blot analysis confirmed that p53 was induced in all cell lines except the shp53 line in which p53 was undetectable both before and after IPTG treatment (Fig. 5B). These results show that cyclin/CDK complexes play a role in the repression of borealin in response to p53 signaling. As a further test of the effect of p53 on borealin, we exposed HCT116 cells to nutlin 3, a small molecule inhibitor of the hDM2-p53 interaction. Nutlin 3 up-regulated p53 in HCT116 cells leading to a rapid down-regulation of the level of borealin protein (Fig. 6A). Borealin was not down-regulated when p53-null HCT116 cells were exposed to nutlin 3 confirming the specificity of this effect (Fig. 6A).
Figure 6. Control of borealin levels by p53, p21/WAF1 and Rb proteins.
Western blotting was used to determine the levels of p53, borealin in the cell lines indicated. β-actin was used to control for protein loading. (A) Effect of p53 on the levels of borealin in cells exposed to nutlin 3. The levels of borealin and p53 in HCT116 p53+/+ and HCT116 p53−/− cells treated with nutlin 3 (10μm) is shown. (B) Effect of p21/WAF1 on the levels of borealin in cells exposed to Nutlin 3. Borealin and p53 were analysed in HCT116 p21+/+ and HCT116 p21−/−cells treated with nutlin 3 (10μm) for the times indicated. (C) Role of Rb family in borealin down regulation in response to DNA damage. Wild type (WT) and p130/107/105Rb-null (TKO) mouse embryonic fibroblasts were treated with adriamycin (ADR; 0.2mg/ml) and analyzed by western blotting using antibodies to borealin and β-Actin as a loading control.
Borealin was repressed more efficiently in wild-type mouse embryo fibroblasts than those lacking p130 and p107 after exposure to adriamycin. This suggested that Rb family proteins are involved in the down-regulation of borealin in response to p53 signaling. Together, these results suggest that the down-regulation of borealin occurs by an indirect, multi-component pathway when p14ARF levels rise. p14ARF up-regulates p53 by blocking its degradation by hDM2. High levels of p53 can induce p21/WAF1 which can inhibit CDKs that would normally inactivate Rb family proteins. One possibility is that poorly phosphorylated Rb proteins bind to the borealin promoter to repress it. Consistent with this idea, a global factor-binding analysis has shown that both p130 and E2F4 are bound to the borealin promoter in quiescent cells in vivo (Cam et al., 2004). It may be assumed that regulation of the human borealin promoter by the Rb/E2F system is responsible for the changes in mRNA levels we have observed. Consistent with this model of regulation, we observed that optimal down-regulation of borealin in response to nutlin 3 was no longer observed in cells lacking p21/WAF1 (Fig. 6B). To further confirm our model of borealin down-regulation, we exposed wild-type MEFs and MEFs lacking all three Rb family proteins (TKO cells) to adriamycin and analysed the level of borealin protein by western blotting. Borealin was down-regulated in both cell types, however the protein was lost more rapidly in the wild-type than the TKO cells (Fig. 6C). This observation indicates that the Rb family participates in down-regulating borealin in response to DNA damage. These results also suggest that an Rb-independent pathway also contributes to borealin down-regulation.
3.5 Alternative splicing of borealin
We used RT-PCR to isolate a cDNA for borealin and obtained two forms differing in splicing of the first intron. To determine if the first intron is frequently left un-spliced, RT-PCRwas used with a forward primer located in the first exon (primer “A”) and a reverse primer in exon 7 (primer “REV”), the combination of which should allow the detection of both spliced and unspliced versions (Fig. 7A). PCR products were separated by using poly-acryla- borealin species (Fig. 7B). A smaller band was observed and appears to represent the spliced version. When RT-PCR reactions were carried out using a forward primer located in intron 1 (primer “B”) and the same reverse primer, a band with a size consistent with the un-spliced mRNA (borealin L) was observed in all 14 cell lines (Fig. 7C). Therefore, the two splice forms are easily detectable. When compared to gapdh as a loading control, it appeared that both mRNAs were found at similar levels in the cell lines examined (Fig. 7C). A kozak consensus in the second exon suggests that the presence of the first intron will not affect the amino acid sequence of the encoded protein. To confirm this idea, cDNAs from both splice versions were transiently transfected into HEK293 cells. Western blotting indicated that the protein produced from both splice variants was equally abundant and of the same apparent molecular weight (Fig. 7D). Interestingly, a higher level of borealin protein was found in transiently transfected cells after they were arrested in mitosis with nocodazole (Fig. 7D). Although our synchronisation experiments indicate higher levels of endogenous borealin mRNA during mitosis this does not explain the effect of nocodazole on ectopic borealin expressed from the constitute CMV promoter which is not cell cycle regulated (Heinrich et al., 2000). Instead, we predict that the abundance of borealin during mitosis is also subject to post-transcriptional control. One possibility is that the degradation of the protein is altered during mitosis. Along these lines, we observed that inhibiting the proteasome caused an increase in the levels of borealin suggesting that it is degraded by this structure (our unpublished observations). Both transcriptional and post-transcriptional mechanisms may be important in allowing the accumulation of sufficient levels of borealin to carry out its functions in chromosome alignment and cytokinesis.
Acknowledgments
We gratefully acknowledge G. Peters for NARF2 cells and B.Vogelstein for p53-null HCT116 cells. This work was supported by a grant from the Concern Foundation to W.R.T.
Abbreviations
- CDK
cyclin dependent kinase
- RT-PCR
reverse transcription-polymerase chain reaction
- EST
expressed sequence tag
- CGAP
cancer genome anatomy project
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agarwal ML, Agarwal A, Taylor WR, Chernova O, Sharma Y, Stark GR. A p53-dependent S-phase checkpoint helps to protect cells from DNA damage in response to starvation for pyrimidine nucleotides. Proc Natl Acad Sci USA. 1998;95:14775–80. doi: 10.1073/pnas.95.25.14775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen MH, thor SP. Survivin--a universal tumor antigen. Histol Histopathol. 2002;17:669–75. doi: 10.14670/HH-17.669. [DOI] [PubMed] [Google Scholar]
- Badie C, Itzhaki JE, Sullivan MJ, Carpenter AJ, Porter AC. Repression of CDK1 and other genes with CDE and CHR promoter elements during DNA damage-induced G(2)/M arrest in human cells. Mol Cell Biol. 2000;20:2358–66. doi: 10.1128/mcb.20.7.2358-2366.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulaire J, Fotedar A, Fotedar R. The functions of the cdk-cyclin kinase inhibitor p21WAF1. Pathol Biol (Paris) 2000;48:190–202. [PubMed] [Google Scholar]
- Brown DC, Gatter KC. Ki67 protein: the immaculate deception? Histopathology. 2002;40:2–11. doi: 10.1046/j.1365-2559.2002.01343.x. [DOI] [PubMed] [Google Scholar]
- Cam H, Balciunaite E, Blais A, Spektor A, Scarpulla RC, Young R, et al. A common set of gene regulatory networks links metabolism and growth inhibition. Mol Cell. 2004;16:399–411. doi: 10.1016/j.molcel.2004.09.037. [DOI] [PubMed] [Google Scholar]
- Chang JL, Chen TH, Wang CF, Chiang YH, Huang YL, Wong FH, et al. Borealin/Dasra B is a cell cycle-regulated chromosomal passenger protein and its nuclear accumulation is linked to poor prognosis for human gastric cancer. Exp Cell Res. 2006;312:962–73. doi: 10.1016/j.yexcr.2005.12.015. [DOI] [PubMed] [Google Scholar]
- Chytil A, Waltner-Law M, West R, Friedman D, Aakre M, Barker D, et al. Construction of a cyclin D1-Cdk2 fusion protein to model the biological functions of cyclin D1-Cdk2 complexes. J Biol Chem. 2004;279:47688–98. doi: 10.1074/jbc.M405938200. [DOI] [PubMed] [Google Scholar]
- Cleveland JL, Sherr CJ. Antagonism of Myc functions by Arf. Cancer Cell. 2004;6:309–11. doi: 10.1016/j.ccr.2004.09.020. [DOI] [PubMed] [Google Scholar]
- Clifford B, Beljin M, Stark GR, Taylor WR. G2 arrest in response to topoisomerase II inhibitors: The role of p53. Cancer Res. 2003;63:4074–4081. [PubMed] [Google Scholar]
- Datta A, Nag A, Pan W, Hay N, Gartel AL, Colamonici O, et al. Myc-ARF (alternate reading frame) interaction inhibits the functions of Myc. J Biol Chem. 2004;279:36698–707. doi: 10.1074/jbc.M312305200. [DOI] [PubMed] [Google Scholar]
- Elledge SJ. Cell cycle checkpoints: preventing an identity crisis. Science. 1996;274:1664–72. doi: 10.1126/science.274.5293.1664. [DOI] [PubMed] [Google Scholar]
- Gassmann R, Carvalho A, Henzing AJ, Ruchaud S, Hudson DF, Honda R, et al. Borealin: a novel chromosomal passenger required for stability of the bipolar mitotic spindle. J Cell Biol. 2004;166:179–91. doi: 10.1083/jcb.200404001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guertin DA, Trautmann S, McCollum D. Cytokinesis in eukaryotes. Microbiol Mol Biol Rev. 2002;66:155–78. doi: 10.1128/MMBR.66.2.155-178.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harbour JW, Dean DC. Rb function in cell-cycle regulation and apoptosis. Nat Cell Biol. 2000;2:E65–7. doi: 10.1038/35008695. [DOI] [PubMed] [Google Scholar]
- Heinrich MC, Silvey KV, Stone S, Zigler AJ, Griffith DJ, Montalto M, et al. Posttranscriptional cell cycle-dependent regulation of human FANCC expression. Blood. 2000;95:3970–7. [PubMed] [Google Scholar]
- Itahana K, Bhat KP, Jin A, Itahana Y, Hawke D, Kobayashi R, et al. Tumor suppressor ARF degrades B23, a nucleolar protein involved in ribosome biogenesis and cell proliferation. Mol Cell. 2003;12:1151–64. doi: 10.1016/s1097-2765(03)00431-3. [DOI] [PubMed] [Google Scholar]
- Jackson MW, Agarwal MK, Yang J, Bruss P, Uchiumi T, Agarwal ML, et al. p130/p107/p105Rb-dependent transcriptional repression during DNA-damage-induced cell-cycle exit at G2. J Cell Sci. 2005;118:1821–32. doi: 10.1242/jcs.02307. [DOI] [PubMed] [Google Scholar]
- Kaur H, Stiff AC, Date DA, Taylor WR. Analysis of mitotic phosphorylation of borealin. BMC Cell Biol. 2007;8:5. doi: 10.1186/1471-2121-8-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein UR, Nigg EA, Gruneberg U. Centromere targeting of the chromosomal passenger complex requires a ternary subcomplex of Borealin, Survivin, and the N-terminal domain of INCENP. Mol Biol Cell. 2006;17:2547–58. doi: 10.1091/mbc.E05-12-1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koffa MD, Casanova CM, Santarella R, Kocher T, Wilm M, Mattaj IW. HURP is part of a Ran-dependent complex involved in spindle formation. Curr Biol. 2006;16:743–54. doi: 10.1016/j.cub.2006.03.056. [DOI] [PubMed] [Google Scholar]
- Molinari M. Cell cycle checkpoints and their inactivation in human cancer. Cell Prolif. 2000;33:261–74. doi: 10.1046/j.1365-2184.2000.00191.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connell MJ, Walworth NC, Carr AM. The G2-phase DNA-damage checkpoint. Trends Cell Biol. 2000;10:296–303. doi: 10.1016/s0962-8924(00)01773-6. [DOI] [PubMed] [Google Scholar]
- Qi Y, Gregory MA, Li Z, Brousal JP, West K, Hann SR. p19ARF directly and differentially controls the functions of c-Myc independently of p53. Nature. 2004;431:712–7. doi: 10.1038/nature02958. [DOI] [PubMed] [Google Scholar]
- Rankin S, Ayad NG, Kirschner MW. Sororin, a substrate of the anaphase-promoting complex, is required for sister chromatid cohesion in vertebrates. Mol Cell. 2005;18:185–200. doi: 10.1016/j.molcel.2005.03.017. [DOI] [PubMed] [Google Scholar]
- Sampath SC, Ohi R, Leismann O, Salic A, Pozniakovski A, Funabiki H. The chromosomal passenger complex is required for chromatin-induced microtubule stabilization and spindle assembly. Cell. 2004;118:187–202. doi: 10.1016/j.cell.2004.06.026. [DOI] [PubMed] [Google Scholar]
- Steeg PS, Zhou Q. Cyclins and breast cancer. Breast Cancer Res Treat. 1998;52:17–28. doi: 10.1023/a:1006102916060. [DOI] [PubMed] [Google Scholar]
- Stott FJ, Bates S, James MC, McConnell BB, Starborg M, Brookes S, et al. The alternative product from the human CDKN2A locus, p14(ARF), participates in a regulatory feedback loop with p53 and MDM2. EMBO J. 1998;17:5001–5014. doi: 10.1093/emboj/17.17.5001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor WR, Stark GR. Regulation of the G2/M transition by p53. Oncogene. 2001;20:1803–1815. doi: 10.1038/sj.onc.1204252. [DOI] [PubMed] [Google Scholar]
- Vagnarelli P, Earnshaw WC. Chromosomal passengers: the four-dimensional regulation of mitotic events. Chromosoma. 2004;113:211–22. doi: 10.1007/s00412-004-0307-3. [DOI] [PubMed] [Google Scholar]
- Vogelstein B, Lane D, Levine AJ. Surfing the p53 network. Nature. 2000;408:307–10. doi: 10.1038/35042675. [DOI] [PubMed] [Google Scholar]
- Whitfield ML, Zheng LX, Baldwin A, Ohta T, Hurt MM, Marzluff WF. Stem-loop binding protein, the protein that binds the 3′ end of histone mRNA, is cell cycle regulated by both translational and posttranslational mechanisms. Mol Cell Biol. 2000;20:4188–98. doi: 10.1128/mcb.20.12.4188-4198.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf G, Hildenbrand R, Schwar C, Grobholz R, Kaufmann M, Stutte HJ, et al. Polo-like kinase: a novel marker of proliferation: correlation with estrogen-receptor expression in human breast cancer. Pathol Res Pract. 2000;196:753–9. doi: 10.1016/S0344-0338(00)80107-7. [DOI] [PubMed] [Google Scholar]
- Wong J, Fang G. HURP controls spindle dynamics to promote proper interkinetochore tension and efficient kinetochore capture. J Cell Biol. 2006;173:879–91. doi: 10.1083/jcb.200511132. [DOI] [PMC free article] [PubMed] [Google Scholar]