Abstract
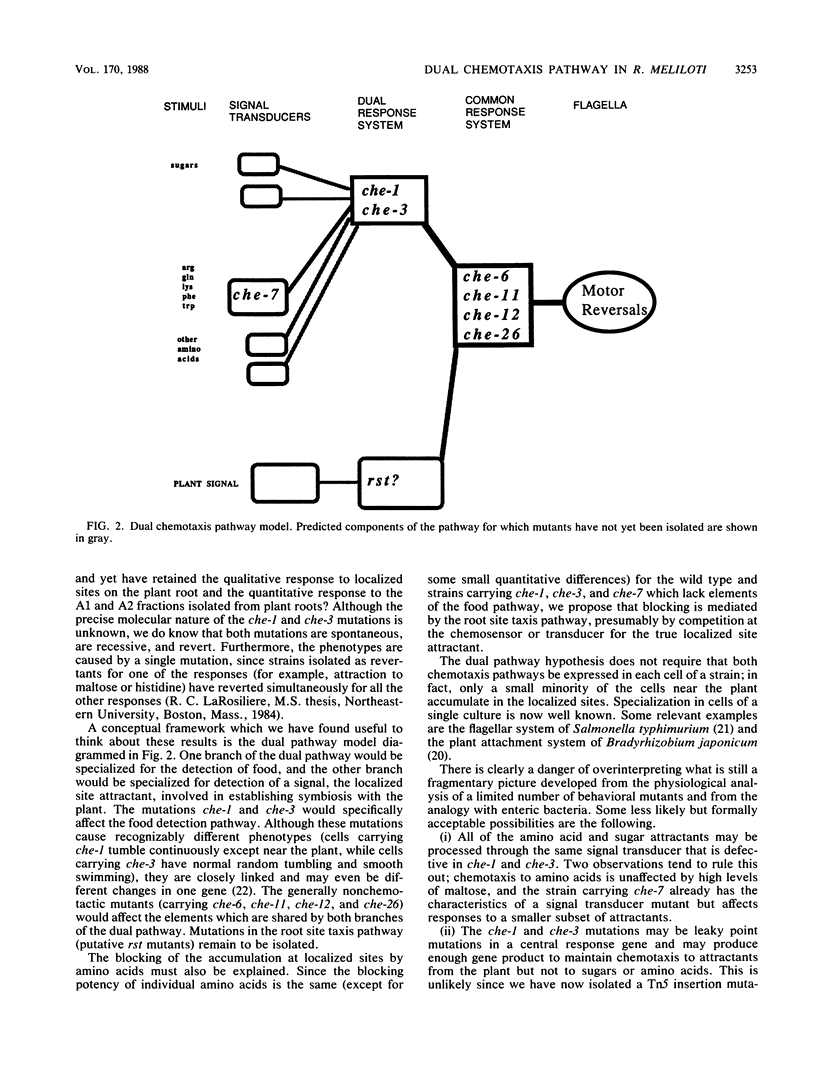
Wild-type and nonchemotactic mutant strains of Rhizobium meliloti were tested for attraction to localized sites on alfalfa roots and for attraction to numerous small molecules, including sugars, amino acids, and two fractions derived from alfalfa root extracts. Four strains (carrying mutations che-6, che-11, che-12, and che-26) lost all responses and were classified as generally nonchemotactic mutants. One strain (carrying mutation che-7) lost responses to a group of structurally unrelated amino acids but retained all other responses and was classified as a putative sensory transducer mutant. Two strains (carrying mutations che-1 and che-3) lost responses to all the amino acids and sugars tested but retained normal responses to localized sites on roots and to the root fractions. These two mutant strains could not be classified according to the generally accepted model for a sensory pathway, derived from studies of enteric bacteria, and provided evidence for a dual chemotaxis pathway in R. meliloti.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler J. A method for measuring chemotaxis and use of the method to determine optimum conditions for chemotaxis by Escherichia coli. J Gen Microbiol. 1973 Jan;74(1):77–91. doi: 10.1099/00221287-74-1-77. [DOI] [PubMed] [Google Scholar]
- Ames P., Bergman K. Competitive advantage provided by bacterial motility in the formation of nodules by Rhizobium meliloti. J Bacteriol. 1981 Nov;148(2):728–p. doi: 10.1128/jb.148.2.728-729.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ames P., Schluederberg S. A., Bergman K. Behavioral mutants of Rhizobium meliloti. J Bacteriol. 1980 Feb;141(2):722–727. doi: 10.1128/jb.141.2.722-727.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Block S. M., Segall J. E., Berg H. C. Impulse responses in bacterial chemotaxis. Cell. 1982 Nov;31(1):215–226. doi: 10.1016/0092-8674(82)90421-4. [DOI] [PubMed] [Google Scholar]
- Boyd A., Simon M. Bacterial chemotaxis. Annu Rev Physiol. 1982;44:501–517. doi: 10.1146/annurev.ph.44.030182.002441. [DOI] [PubMed] [Google Scholar]
- Caetano-Anollés G., Wall L. G., De Micheli A. T., Macchi E. M., Bauer W. D., Favelukes G. Role of Motility and Chemotaxis in Efficiency of Nodulation by Rhizobium meliloti. Plant Physiol. 1988 Apr;86(4):1228–1235. doi: 10.1104/pp.86.4.1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finan T. M., Hartweig E., LeMieux K., Bergman K., Walker G. C., Signer E. R. General transduction in Rhizobium meliloti. J Bacteriol. 1984 Jul;159(1):120–124. doi: 10.1128/jb.159.1.120-124.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulash M., Ames P., Larosiliere R. C., Bergman K. Rhizobia are attracted to localized sites on legume roots. Appl Environ Microbiol. 1984 Jul;48(1):149–152. doi: 10.1128/aem.48.1.149-152.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi T., Tsuchiya H., Naruse H. Reversed-phase ion-pair chromatography of amino acids. Application to the determination of amino acids in plasma samples and dried blood on filter papers. J Chromatogr. 1983 May 13;274:318–324. [PubMed] [Google Scholar]
- Hedblom M. L., Adler J. Chemotactic response of Escherichia coli to chemically synthesized amino acids. J Bacteriol. 1983 Sep;155(3):1463–1466. doi: 10.1128/jb.155.3.1463-1466.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langer R., Fefferman M., Gryska P., Bergman K. A simple method for studying chemotaxis using sustained release of attractants from inert polymers. Can J Microbiol. 1980 Feb;26(2):274–278. doi: 10.1139/m80-045. [DOI] [PubMed] [Google Scholar]
- Leigh J. A., Signer E. R., Walker G. C. Exopolysaccharide-deficient mutants of Rhizobium meliloti that form ineffective nodules. Proc Natl Acad Sci U S A. 1985 Sep;82(18):6231–6235. doi: 10.1073/pnas.82.18.6231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Napoli C., Albersheim P. Infection and nodulation of clover by nonmotile Rhizobium trifolii. J Bacteriol. 1980 Feb;141(2):979–980. doi: 10.1128/jb.141.2.979-980.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niwano M., Taylor B. L. Novel sensory adaptation mechanism in bacterial chemotaxis to oxygen and phosphotransferase substrates. Proc Natl Acad Sci U S A. 1982 Jan;79(1):11–15. doi: 10.1073/pnas.79.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spudich J. L., Koshland D. E., Jr Quantitation of the sensory response in bacterial chemotaxis. Proc Natl Acad Sci U S A. 1975 Feb;72(2):710–713. doi: 10.1073/pnas.72.2.710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vesper S. J., Bauer W. D. Role of Pili (Fimbriae) in Attachment of Bradyrhizobium japonicum to Soybean Roots. Appl Environ Microbiol. 1986 Jul;52(1):134–141. doi: 10.1128/aem.52.1.134-141.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler R. J., Peirce C., Bergman K. Mapping and cloning of a fla-che region of the Rhizobium meliloti chromosome. J Bacteriol. 1986 Nov;168(2):785–790. doi: 10.1128/jb.168.2.785-790.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]