Abstract
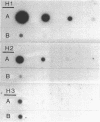
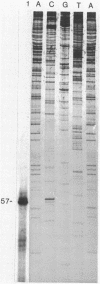
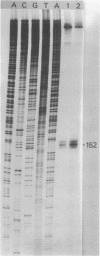
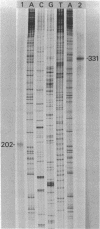
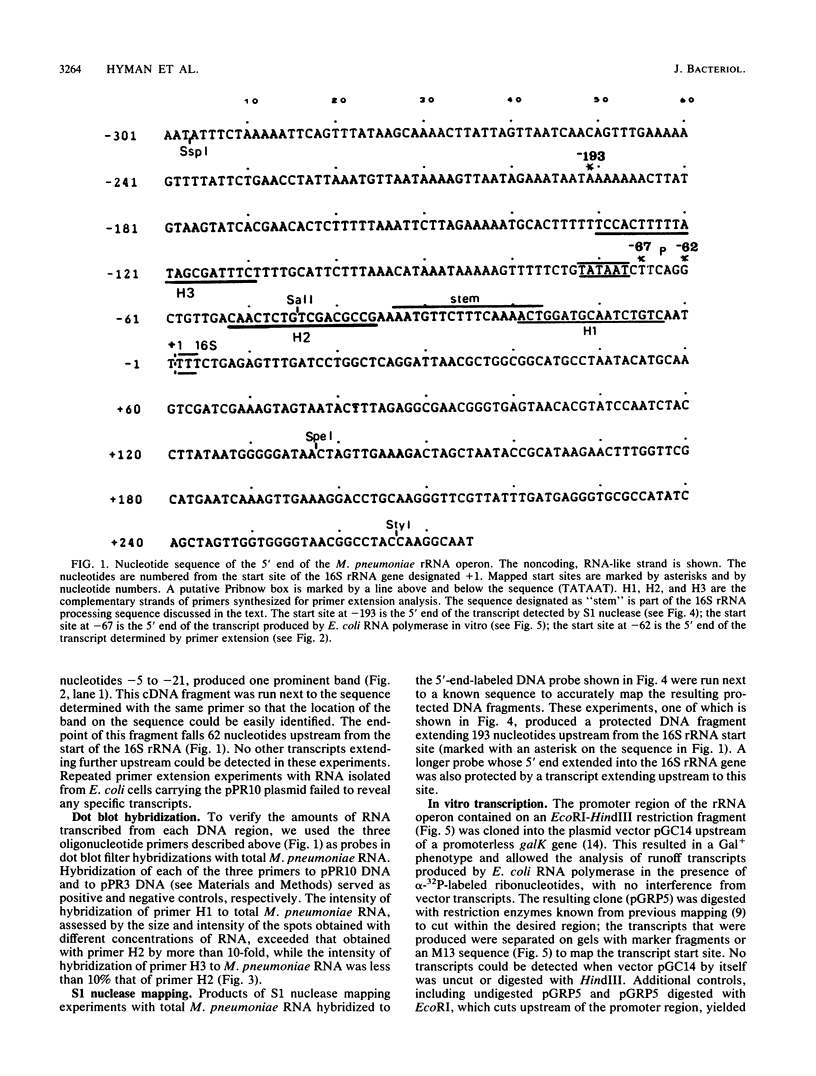
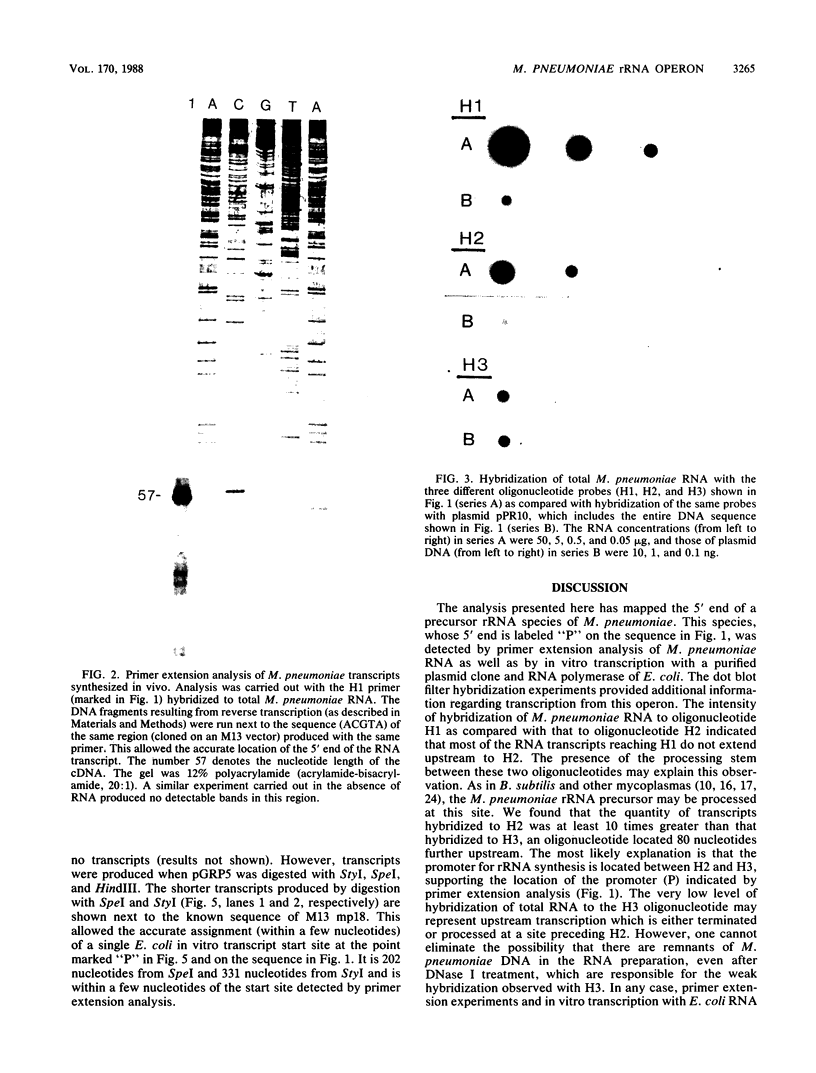
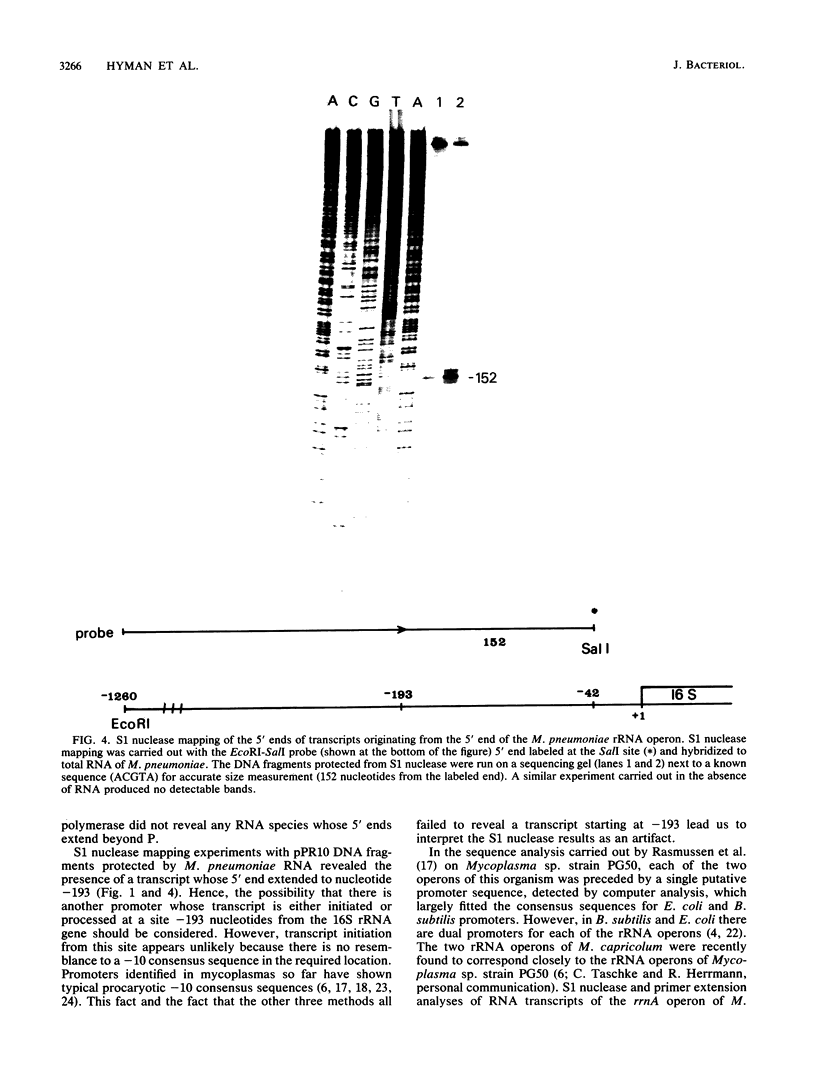
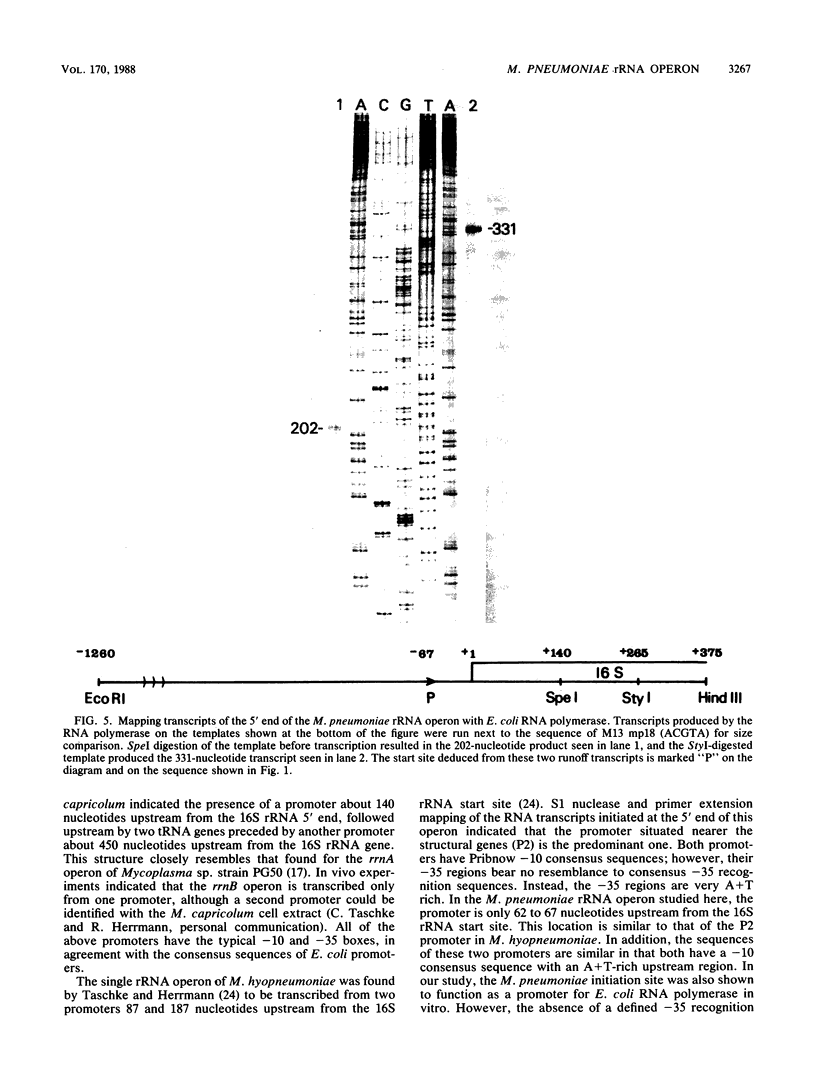
RNA transcripts starting from the 5' end of the single Mycoplasma pneumoniae rRNA operon were analyzed by several methods. By primer extension analysis a start site was found 62 nucleotides upstream from the start site of the 16S rRNA. This site was preceded by a putative Pribnow box; however, a defined -35 recognition region was absent. The cloned rRNA operon was transcribed in vitro by using purified RNA polymerase of Escherichia coli. A single start site could be demonstrated within a few nucleotides of the start site found by primer extension analysis of M. pneumoniae transcripts. When fragments from the cloned operon were used as hybridization probes, S1 nuclease mapping yielded a single transcript extending approximately 193 nucleotides upstream from the 16S rRNA start site. The region surrounding this endpoint did not resemble any known promoter sequence. Dot blot hybridization of M. pneumoniae RNA to three oligonucleotides consisting of nucleotides -5 to -21, -38 to -54, and -112 to -132 (from the start of the 16S rRNA gene) indicated that most rRNA transcripts were processed at the stem site preceding the 16S rRNA gene. The majority of the longer precursor transcripts, extending beyond this point, did not extend further upstream to an oligonucleotide consisting of nucleotides -112 to -132. It was concluded that transcription of the rRNA operon of M. pneumoniae is initiated by a single promoter. The nucleotide sequence of the region is presented.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amikam D., Glaser G., Razin S. Mycoplasmas (Mollicutes) have a low number of rRNA genes. J Bacteriol. 1984 Apr;158(1):376–378. doi: 10.1128/jb.158.1.376-378.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berk A. J., Sharp P. A. Sizing and mapping of early adenovirus mRNAs by gel electrophoresis of S1 endonuclease-digested hybrids. Cell. 1977 Nov;12(3):721–732. doi: 10.1016/0092-8674(77)90272-0. [DOI] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gafny R., Hyman H. C., Razin S., Glaser G. Promoters of Mycoplasma capricolum ribosomal RNA operons: identical activities but different regulation in homologous and heterologous cells. Nucleic Acids Res. 1988 Jan 11;16(1):61–76. doi: 10.1093/nar/16.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaser G., Razin A., Razin S. Stable RNA synthesis and its control in Mycoplasma capricolum. Nucleic Acids Res. 1981 Aug 11;9(15):3641–3646. doi: 10.1093/nar/9.15.3641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henikoff S. Unidirectional digestion with exonuclease III creates targeted breakpoints for DNA sequencing. Gene. 1984 Jun;28(3):351–359. doi: 10.1016/0378-1119(84)90153-7. [DOI] [PubMed] [Google Scholar]
- Hyman H. C., Gafny R., Glaser G., Razin S. Transcription control elements of the Mycoplasma pneumoniae rRNA operon. Isr J Med Sci. 1987 Jun;23(6):585–590. [PubMed] [Google Scholar]
- Iwami M., Muto A., Yamao F., Osawa S. Nucleotide sequence of the rrnB 16S ribosomal RNA gene from Mycoplasma capricolum. Mol Gen Genet. 1984;196(2):317–322. doi: 10.1007/BF00328065. [DOI] [PubMed] [Google Scholar]
- Kawauchi Y., Muto A., Yamao F., Osawa S. Molecular cloning of ribosomal protein genes from Mycoplasma capricolum. Mol Gen Genet. 1984;196(3):521–525. doi: 10.1007/BF00436202. [DOI] [PubMed] [Google Scholar]
- McKenney K., Shimatake H., Court D., Schmeissner U., Brady C., Rosenberg M. A system to study promoter and terminator signals recognized by Escherichia coli RNA polymerase. Gene Amplif Anal. 1981;2:383–415. [PubMed] [Google Scholar]
- Mouchès C., Candresse T., Barroso G., Saillard C., Wroblewski H., Bové J. M. Gene for spiralin, the major membrane protein of the helical mollicute Spiroplasma citri: cloning and expression in Escherichia coli. J Bacteriol. 1985 Dec;164(3):1094–1099. doi: 10.1128/jb.164.3.1094-1099.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogasawara N., Moriya S., Yoshikawa H. Structure and organization of rRNA operons in the region of the replication origin of the Bacillus subtilis chromosome. Nucleic Acids Res. 1983 Sep 24;11(18):6301–6318. doi: 10.1093/nar/11.18.6301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razin S. Molecular biology and genetics of mycoplasmas (Mollicutes). Microbiol Rev. 1985 Dec;49(4):419–455. doi: 10.1128/mr.49.4.419-455.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salser W., Gesteland R. F., Bolle A. In vitro synthesis of bacteriophage lysozyme. Nature. 1967 Aug 5;215(5101):588–591. doi: 10.1038/215588a0. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart G. C., Bott K. F. DNA sequence of the tandem ribosomal RNA promoter for B. subtilis operon rrnB. Nucleic Acids Res. 1983 Sep 24;11(18):6289–6300. doi: 10.1093/nar/11.18.6289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su C. J., Tryon V. V., Baseman J. B. Cloning and sequence analysis of cytadhesin P1 gene from Mycoplasma pneumoniae. Infect Immun. 1987 Dec;55(12):3023–3029. doi: 10.1128/iai.55.12.3023-3029.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taschke C., Herrmann R. Analysis of transcription and processing signals of the 16S-23S rRNA operon of Mycoplasma hyopneumoniae. Mol Gen Genet. 1986 Dec;205(3):434–441. doi: 10.1007/BF00338079. [DOI] [PubMed] [Google Scholar]
- Taschke C., Ruland K., Herrmann R. Nucleotide sequence of the 16S rRNA of Mycoplasma hyopneumoniae. Nucleic Acids Res. 1987 May 11;15(9):3918–3918. doi: 10.1093/nar/15.9.3918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tully J. G., Whitcomb R. F., Clark H. F., Williamson D. L. Pathogenic mycoplasmas: cultivation and vertebrate pathogenicity of a new spiroplasma. Science. 1977 Mar 4;195(4281):892–894. doi: 10.1126/science.841314. [DOI] [PubMed] [Google Scholar]
- Wise K. S. Antigen expression from cloned genes of Mycoplasma hyorhinis: an approach to mycoplasma genomic analysis. Isr J Med Sci. 1984 Sep;20(9):754–757. [PubMed] [Google Scholar]
- Woese C. R., Gutell R., Gupta R., Noller H. F. Detailed analysis of the higher-order structure of 16S-like ribosomal ribonucleic acids. Microbiol Rev. 1983 Dec;47(4):621–669. doi: 10.1128/mr.47.4.621-669.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woese C. R., Maniloff J., Zablen L. B. Phylogenetic analysis of the mycoplasmas. Proc Natl Acad Sci U S A. 1980 Jan;77(1):494–498. doi: 10.1073/pnas.77.1.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woese C. R., Stackebrandt E., Ludwig W. What are mycoplasmas: the relationship of tempo and mode in bacterial evolution. J Mol Evol. 1984;21(4):305–316. doi: 10.1007/BF02115648. [DOI] [PubMed] [Google Scholar]