Abstract
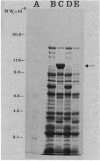
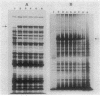
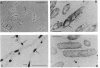
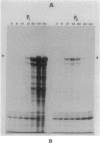
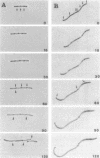
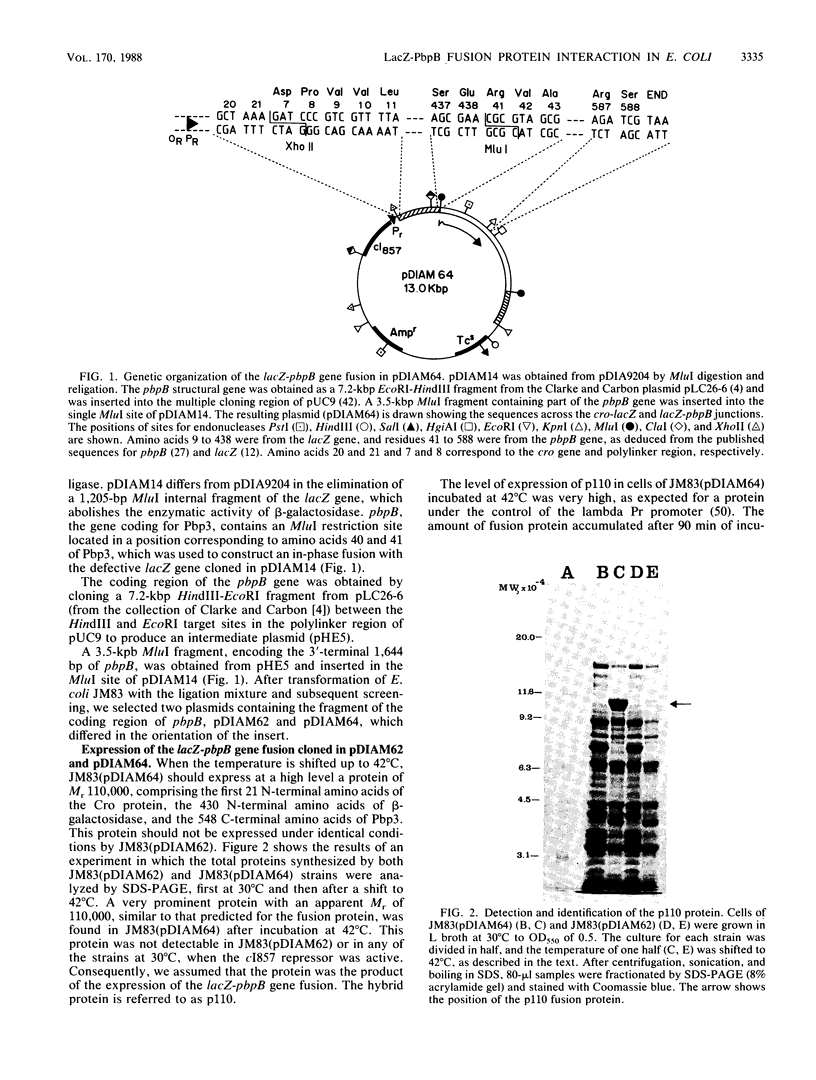
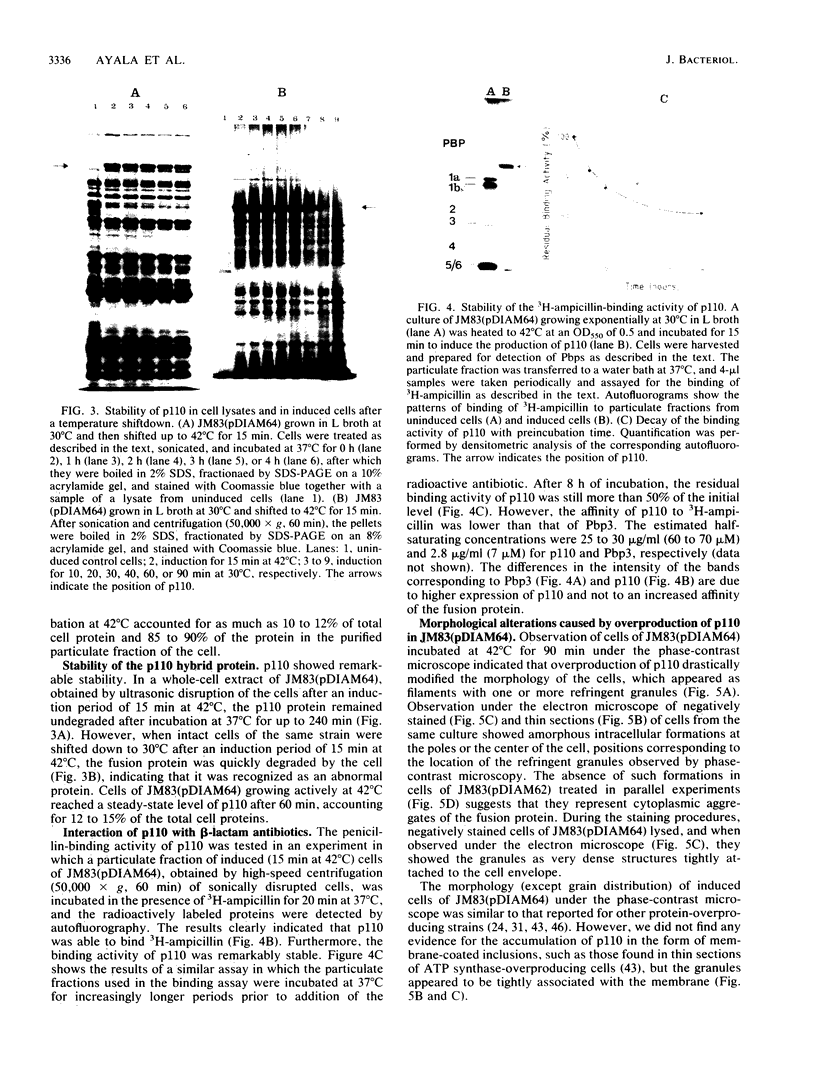
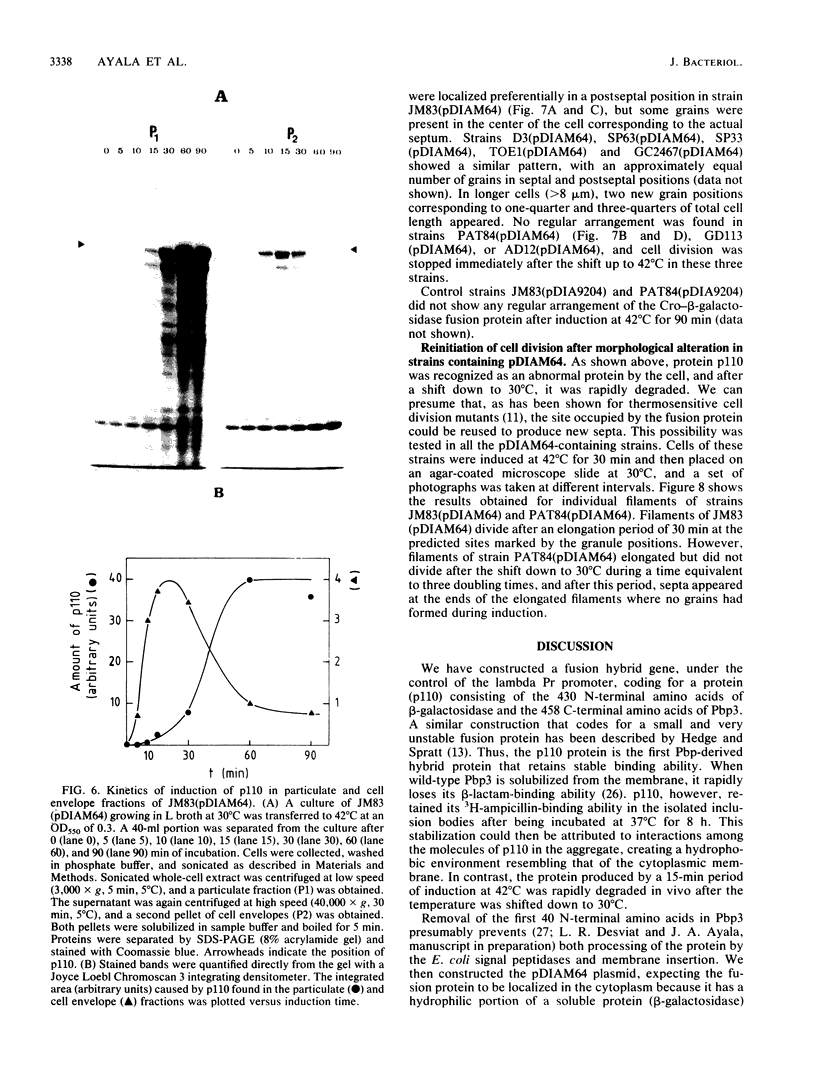
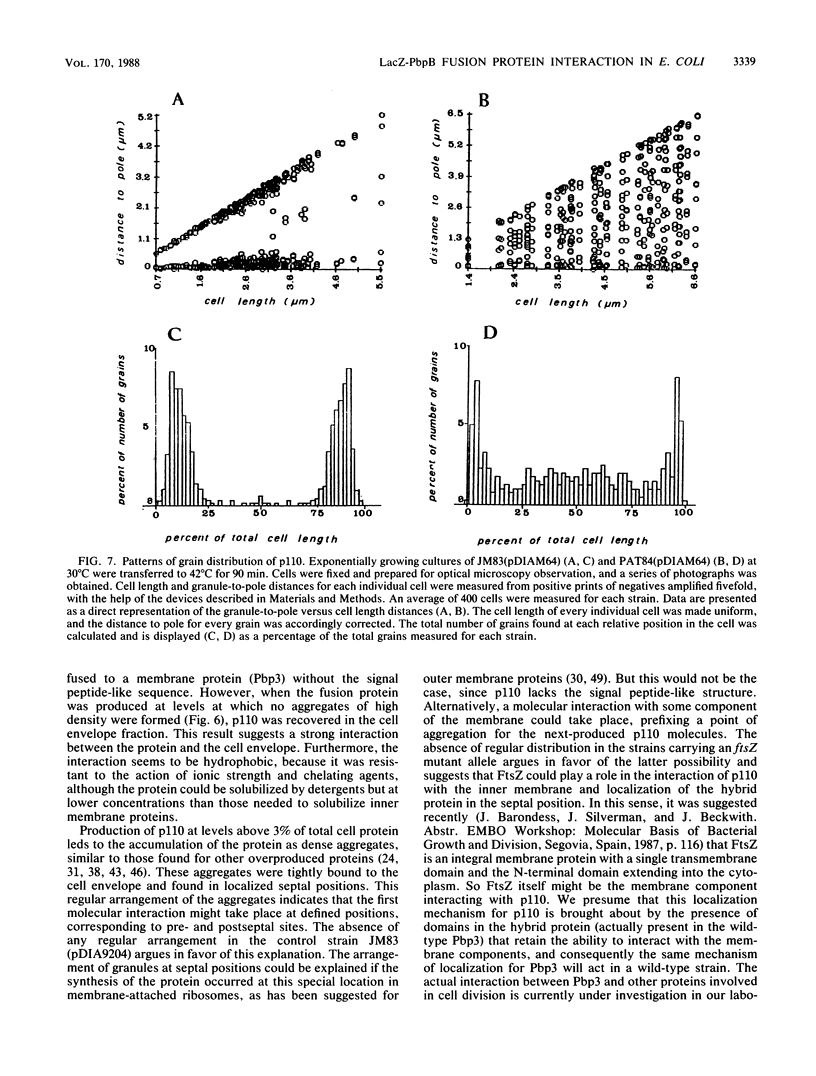
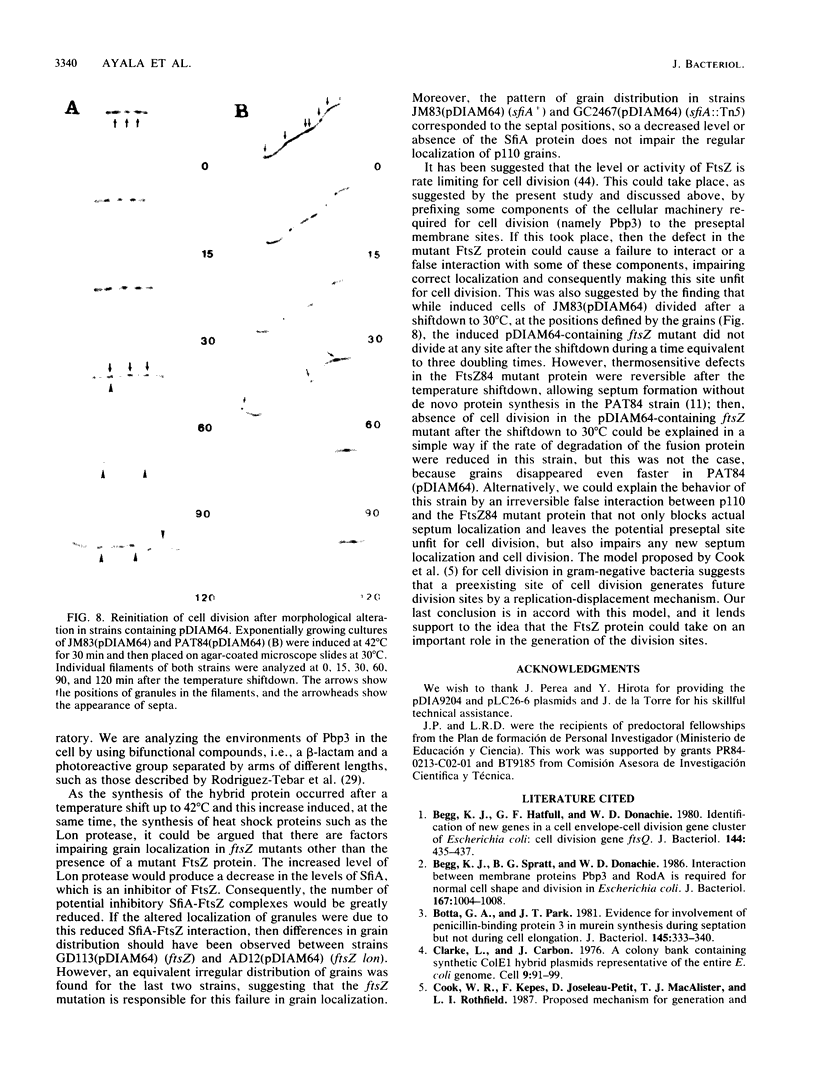
An in-phase gene fusion consisting of the 5'-terminal 1,314 base pairs (bp) of the structural gene for beta-galactosidase (lacZ) and the 3'-terminal 1,644 bp of the structural gene coding for penicillin-binding protein 3 (pbpB) of Escherichia coli was constructed and cloned in the plasmid pDIAM64. The product of the fusion gene was a remarkably stable protein with an apparent molecular weight of 110,000 (p110) that retained the ability to covalently interact with beta-lactam antibiotics. The fusion protein was found associated with the membrane at low levels of induction, but it accumulated in the cytoplasm of cells induced for a long time as inclusion bodies of high density. Inclusion bodies were localized at defined positions corresponding to septal sites in all of the pDIAM64-containing strains tested except PAT84 and GD113 (which carry the ftsZ84 mutant allele). These findings indicate a possible role of the FtsZ protein in the integration of Pbp3 into the membrane and in septum localization during the cell division cycle.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Begg K. J., Hatfull G. F., Donachie W. D. Identification of new genes in a cell envelope-cell division gene cluster of Escherichia coli: cell division gene ftsQ. J Bacteriol. 1980 Oct;144(1):435–437. doi: 10.1128/jb.144.1.435-437.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begg K. J., Spratt B. G., Donachie W. D. Interaction between membrane proteins PBP3 and rodA is required for normal cell shape and division in Escherichia coli. J Bacteriol. 1986 Sep;167(3):1004–1008. doi: 10.1128/jb.167.3.1004-1008.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botta G. A., Park J. T. Evidence for involvement of penicillin-binding protein 3 in murein synthesis during septation but not during cell elongation. J Bacteriol. 1981 Jan;145(1):333–340. doi: 10.1128/jb.145.1.333-340.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke L., Carbon J. A colony bank containing synthetic Col El hybrid plasmids representative of the entire E. coli genome. Cell. 1976 Sep;9(1):91–99. doi: 10.1016/0092-8674(76)90055-6. [DOI] [PubMed] [Google Scholar]
- Cook W. R., Kepes F., Joseleau-Petit D., MacAlister T. J., Rothfield L. I. Proposed mechanism for generation and localization of new cell division sites during the division cycle of Escherichia coli. Proc Natl Acad Sci U S A. 1987 Oct;84(20):7144–7148. doi: 10.1073/pnas.84.20.7144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Ari R., Huisman O. Novel mechanism of cell division inhibition associated with the SOS response in Escherichia coli. J Bacteriol. 1983 Oct;156(1):243–250. doi: 10.1128/jb.156.1.243-250.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Descoteaux A., Drapeau G. R. Regulation of cell division in Escherichia coli K-12: probable interactions among proteins FtsQ, FtsA, and FtsZ. J Bacteriol. 1987 May;169(5):1938–1942. doi: 10.1128/jb.169.5.1938-1942.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dev I. K., Ray P. H. Rapid assay and purification of a unique signal peptidase that processes the prolipoprotein from Escherichia coli B. J Biol Chem. 1984 Sep 10;259(17):11114–11120. [PubMed] [Google Scholar]
- Donachie W. D., Begg K. J., Lutkenhaus J. F., Salmond G. P., Martinez-Salas E., Vincente M. Role of the ftsA gene product in control of Escherichia coli cell division. J Bacteriol. 1979 Nov;140(2):388–394. doi: 10.1128/jb.140.2.388-394.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donachie W. D., Begg K. J., Vicente M. Cell length, cell growth and cell division. Nature. 1976 Nov 25;264(5584):328–333. doi: 10.1038/264328a0. [DOI] [PubMed] [Google Scholar]
- Fowler A. V., Zabin I. The amino acid sequence of beta-galactosidase of Escherichia coli. Proc Natl Acad Sci U S A. 1977 Apr;74(4):1507–1510. doi: 10.1073/pnas.74.4.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedge P. J., Spratt B. G. A gene fusion that localises the penicillin-binding domain of penicillin-binding protein 3 of Escherichia coli. FEBS Lett. 1984 Oct 15;176(1):179–184. doi: 10.1016/0014-5793(84)80936-9. [DOI] [PubMed] [Google Scholar]
- Ishino F., Matsuhashi M. Peptidoglycan synthetic enzyme activities of highly purified penicillin-binding protein 3 in Escherichia coli: a septum-forming reaction sequence. Biochem Biophys Res Commun. 1981 Aug 14;101(3):905–911. doi: 10.1016/0006-291x(81)91835-0. [DOI] [PubMed] [Google Scholar]
- LENNOX E. S. Transduction of linked genetic characters of the host by bacteriophage P1. Virology. 1955 Jul;1(2):190–206. doi: 10.1016/0042-6822(55)90016-7. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K., Favre M. Maturation of the head of bacteriophage T4. I. DNA packaging events. J Mol Biol. 1973 Nov 15;80(4):575–599. doi: 10.1016/0022-2836(73)90198-8. [DOI] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Quantitative film detection of 3H and 14C in polyacrylamide gels by fluorography. Eur J Biochem. 1975 Aug 15;56(2):335–341. doi: 10.1111/j.1432-1033.1975.tb02238.x. [DOI] [PubMed] [Google Scholar]
- Leplatois P., Danchin A. Vectors for high conditional expression of cloned genes. Biochimie. 1983 Jun;65(6):317–324. doi: 10.1016/s0300-9084(83)80153-9. [DOI] [PubMed] [Google Scholar]
- Little J. W., Mount D. W. The SOS regulatory system of Escherichia coli. Cell. 1982 May;29(1):11–22. doi: 10.1016/0092-8674(82)90085-x. [DOI] [PubMed] [Google Scholar]
- Lutkenhaus J. F., Wolf-Watz H., Donachie W. D. Organization of genes in the ftsA-envA region of the Escherichia coli genetic map and identification of a new fts locus (ftsZ). J Bacteriol. 1980 May;142(2):615–620. doi: 10.1128/jb.142.2.615-620.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutkenhaus J., Sanjanwala B., Lowe M. Overproduction of FtsZ suppresses sensitivity of lon mutants to division inhibition. J Bacteriol. 1986 Jun;166(3):756–762. doi: 10.1128/jb.166.3.756-762.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuzawa H., Datta P., Matsuhashi M. Behavior of penicillin-binding proteins in Escherichia coli upon heat and detergent treatments and partial purification of penicillin-binding proteins 1A and 1B. J Bacteriol. 1979 Jun;138(3):1029–1032. doi: 10.1128/jb.138.3.1029-1032.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messing J., Crea R., Seeburg P. H. A system for shotgun DNA sequencing. Nucleic Acids Res. 1981 Jan 24;9(2):309–321. doi: 10.1093/nar/9.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura M., Maruyama I. N., Soma M., Kato J., Suzuki H., Horota Y. On the process of cellular division in Escherichia coli: nucleotide sequence of the gene for penicillin-binding protein 3. Mol Gen Genet. 1983;191(1):1–9. doi: 10.1007/BF00330881. [DOI] [PubMed] [Google Scholar]
- Ray P., Dev I., MacGregor C., Bassford P., Jr Signal peptidases. Curr Top Microbiol Immunol. 1986;125:75–102. doi: 10.1007/978-3-642-71251-7_7. [DOI] [PubMed] [Google Scholar]
- Rodríguez-Tébar A., Arán V., Vázquez D. Labelling and cross-linking of Escherichia coli penicillin-binding proteins with bis-beta-lactam antibiotics. Eur J Biochem. 1984 Mar 1;139(2):287–293. doi: 10.1111/j.1432-1033.1984.tb08006.x. [DOI] [PubMed] [Google Scholar]
- Ryter A., Shuman H., Schwartz M. Intergration of the receptor for bacteriophage lambda in the outer membrane of Escherichia coli: coupling with cell division. J Bacteriol. 1975 Apr;122(1):295–301. doi: 10.1128/jb.122.1.295-301.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silhavy T. J., Beckwith J. R. Uses of lac fusions for the study of biological problems. Microbiol Rev. 1985 Dec;49(4):398–418. doi: 10.1128/mr.49.4.398-418.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spratt B. G. Distinct penicillin binding proteins involved in the division, elongation, and shape of Escherichia coli K12. Proc Natl Acad Sci U S A. 1975 Aug;72(8):2999–3003. doi: 10.1073/pnas.72.8.2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spratt B. G. Penicillin-binding proteins and the future of beta-lactam antibiotics. The Seventh Fleming Lecture. J Gen Microbiol. 1983 May;129(5):1247–1260. doi: 10.1099/00221287-129-5-1247. [DOI] [PubMed] [Google Scholar]
- Spratt B. G. Temperature-sensitive cell division mutants of Escherichia coli with thermolabile penicillin-binding proteins. J Bacteriol. 1977 Jul;131(1):293–305. doi: 10.1128/jb.131.1.293-305.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki H., Nishimura Y., Hirota Y. On the process of cellular division in Escherichia coli: a series of mutants of E. coli altered in the penicillin-binding proteins. Proc Natl Acad Sci U S A. 1978 Feb;75(2):664–668. doi: 10.1073/pnas.75.2.664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tommassen J., Leunissen J., van Damme-Jongsten M., Overduin P. Failure of E. coli K-12 to transport PhoE-LacZ hybrid proteins out of the cytoplasm. EMBO J. 1985 Apr;4(4):1041–1047. doi: 10.1002/j.1460-2075.1985.tb03736.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tormo A., Ayala J. A., de Pedro M. A., Aldea M., Vicente M. Interaction of FtsA and PBP3 proteins in the Escherichia coli septum. J Bacteriol. 1986 Jun;166(3):985–992. doi: 10.1128/jb.166.3.985-992.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tormo A., Martínez-Salas E., Vicente M. Involvement of the ftsA gene product in late stages of the Escherichia coli cell cycle. J Bacteriol. 1980 Feb;141(2):806–813. doi: 10.1128/jb.141.2.806-813.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuomanen E. Newly made enzymes determine ongoing cell wall synthesis and the antibacterial effects of cell wall synthesis inhibitors. J Bacteriol. 1986 Aug;167(2):535–543. doi: 10.1128/jb.167.2.535-543.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira J., Messing J. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982 Oct;19(3):259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]
- Ward J. E., Jr, Lutkenhaus J. Overproduction of FtsZ induces minicell formation in E. coli. Cell. 1985 Oct;42(3):941–949. doi: 10.1016/0092-8674(85)90290-9. [DOI] [PubMed] [Google Scholar]
- Wientjes F. B., Olijhoek T. J., Schwarz U., Nanninga N. Labeling pattern of major penicillin-binding proteins of Escherichia coli during the division cycle. J Bacteriol. 1983 Mar;153(3):1287–1293. doi: 10.1128/jb.153.3.1287-1293.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams D. C., Van Frank R. M., Muth W. L., Burnett J. P. Cytoplasmic inclusion bodies in Escherichia coli producing biosynthetic human insulin proteins. Science. 1982 Feb 5;215(4533):687–689. doi: 10.1126/science.7036343. [DOI] [PubMed] [Google Scholar]
- Woldringh C. L., de Jong M. A., van den Berg W., Koppes L. Morphological analysis of the division cycle of two Escherichia coli substrains during slow growth. J Bacteriol. 1977 Jul;131(1):270–279. doi: 10.1128/jb.131.1.270-279.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfe P. B., Silver P., Wickner W. The isolation of homogeneous leader peptidase from a strain of Escherichia coli which overproduces the enzyme. J Biol Chem. 1982 Jul 10;257(13):7898–7902. [PubMed] [Google Scholar]
- Yamada H., Nogami T., Mizushima S. Arrangement of bacteriophage lambda receptor protein (LamB) in the cell surface of Escherichia coli: a reconstitution study. J Bacteriol. 1981 Aug;147(2):660–669. doi: 10.1128/jb.147.2.660-669.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zabeau M., Stanley K. K. Enhanced expression of cro-beta-galactosidase fusion proteins under the control of the PR promoter of bacteriophage lambda. EMBO J. 1982;1(10):1217–1224. doi: 10.1002/j.1460-2075.1982.tb00016.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Meyenburg K., Jørgensen B. B., van Deurs B. Physiological and morphological effects of overproduction of membrane-bound ATP synthase in Escherichia coli K-12. EMBO J. 1984 Aug;3(8):1791–1797. doi: 10.1002/j.1460-2075.1984.tb02047.x. [DOI] [PMC free article] [PubMed] [Google Scholar]