Abstract
Regulation of circadian clock components by phosphorylation plays essential roles in clock functions and is conserved from fungi to mammals. In the Neurospora circadian negative feedback loop, FREQUENCY (FRQ) protein inhibits WHITE COLLAR (WC) complex activity by recruiting the casein kinases CKI and CKII to phosphorylate the WC proteins, resulting in the repression of frq transcription. On the other hand, CKI and CKII progressively phosphorylate FRQ to promote FRQ degradation, a process that is a major determinant of circadian period length. Here, by using whole-cell isotope labeling and quantitative mass spectrometry methods, we show that the WC-1 phosphorylation events critical for the negative feedback process occur sequentially—first by a priming kinase, then by the FRQ-recruited casein kinases. We further show that the cyclic AMP-dependent protein kinase A (PKA) is essential for clock function and inhibits WC activity by serving as a priming kinase for the casein kinases. In addition, PKA also regulates FRQ phosphorylation, but unlike CKI and CKII, PKA stabilizes FRQ, similar to the stabilization of human PERIOD2 (hPER2) due to the phosphorylation at the familial advanced sleep phase syndrome (FASPS) site. Thus, PKA is a key clock component that regulates several critical processes in the circadian negative feedback loop.
[Keywords: Circadian clock, Neurospora, protein kinase A, phosphorylation, casein kinase I]
The autoregulatory negative feedback loops controlling the eukaryotic circadian clocks consist of positive and negative elements (Dunlap 1999; King and Takahashi 2000; Allada et al. 2001; Reppert and Weaver 2001; Young and Kay 2001; Sehgal 2004). Despite the evolutionary distances among eukaryotic organisms, the circadian clock mechanisms from fungi to mammals share remarkable similarities (Liu and Bell-Pedersen 2006; Heintzen and Liu 2007). In the filamentous fungus Neurospora circadian negative feedback loop, there are four core components: WHITE COLLAR-1 (WC-1), WC-2, FREQUENCY (FRQ), and a FRQ-interacting RNA helicase FRH (Dunlap 2006). Like their counterparts in the animal clocks, WC-1 and WC-2 are PAS domain-containing transcription factors that form a heteromeric WC complex (WCC). In the dark, WCC activates the transcription of the frq gene by binding to the Clock box (C-box) of frq promoter (Crosthwaite et al. 1997; Cheng et al. 2001b, 2002; Loros and Dunlap 2001; Froehlich et al. 2003; He and Liu 2005; He et al. 2006; Belden et al. 2007). After the synthesis of FRQ protein, FRQ self-dimerizes and forms a complex (called FFC) with FRH (Cheng et al. 2001a, 2005). FFC represses the transcription of frq by inhibiting WCC activity through their physical interaction (Aronson et al. 1994; Merrow et al. 1997, 2001; Cheng et al. 2001a, 2003; Denault et al. 2001; Froehlich et al. 2003; He et al. 2006). This circadian negative feedback loop generates the robust circadian rhythms of frq RNA and FRQ protein in constant darkness (DD) (Garceau et al. 1997).
Post-translational modification of clock proteins by phosphorylation plays essential roles in all circadian clocks (Price et al. 1998; Lowrey et al. 2000; Lin et al. 2002; Sathyanarayanan et al. 2004; Liu 2005; Nakajima et al. 2005; Mori et al. 2007). In eukaryotic organisms, the post-translational mechanisms regulating circadian clocks are remarkably conserved from Neurospora to mammals (Liu and Bell-Pedersen 2006; Heintzen and Liu 2007). The importance and conservation of the eukaryotic circadian systems are highlighted by the fact that either a mutation in human PERIOD2 (hPER2) at one of its phosphorylation sites or a mutation of casein kinase I (CKI) δ causes familial advanced sleep phase syndrome (FASPS) (Toh et al. 2001; Xu et al. 2005).
In Neurospora, WC-1, WC-2, and FRQ are regulated by phosphorylation. The phosphorylation of WC plays an essential role in the negative feedback loop process. WC phosphorylation inhibits WCC activity and both WC-1 and WC-2 are hypophosphorylated in the frq-null strain, suggesting that FRQ inhibits WCC activity by promoting WC phosphorylation (He and Liu 2005; He et al. 2005b; Schafmeier et al. 2005). Our recent results indicated that FRQ accomplishes its transcription repressor function by recruiting CK-1a (the homolog of human CKI δ/ε) and CKII to mediate the FRQ-dependent WC phosphorylation (He et al. 2006). In Neurospora mutants of ck-1a and the catalytic subunit of CKII (cka), WCs are hypophosphorylated and the WCC activity cannot be inhibited by FRQ. A similar model was also proposed in Drosophila (Nawathean and Rosbash 2004; Kim and Edery 2006; Yu et al. 2006; Kim et al. 2007), suggesting a conserved mechanism for the negative feedback process in eukaryotic circadian clocks. The phosphorylation events mediated by CKI and CKII to inhibit WCC activity are not known.
FRQ is progressively phosphorylated by CK-1a, CKII, and CAMK-1 (Garceau et al. 1997; Gorl et al. 2001; Yang et al. 2001, 2002, 2003; He et al. 2006). Protein phosphatases PP1 and PP2A counter the action of the kinases to regulate FRQ phosphorylation status (Yang et al. 2004). The extensively phosphorylated FRQ is then targeted for degradation through the ubiquitin–proteasome pathway mediated by ubiquitin E3 ligase SCFFWD-1 (He et al. 2003, 2005a). In ck-1a and cka mutants, FRQ is hypophosphorylated and more stable relative to wild-type strain, indicating that a major role of FRQ phosphorylation by CK-1a and CKII is to promote FRQ degradation. This phosphorylation-dependent degradation pathway is conserved from Neurospora to mammals (He et al. 2006). In Neurospora, the FRQ phosphorylation–degradation pathway is a major determinant of the circadian period length (Liu et al. 2000; Yang et al. 2003).
Although degradation has been shown to be a major function of FRQ and animal PER phosphorylation, recent studies of hPER2 phosphorylation at the FASPS site suggest that this hPER2 phosphorylation leads to the stabilization of the protein and increase of PER2 transcription (Vanselow et al. 2006; Xu et al. 2007). Thus, phosphorylation of PER2 at different sites appears to have opposite roles in regulating its stability. In addition, the phosphorylation of hPER2 near the FASPS site occurs sequentially. The initial phosphorylation of the FASPS mutation site (S662) facilitates the phosphorylation of downstream sites by CKI δ/ε. However, CKI cannot phosphorylate S662, indicating the existence of an unidentified kinase that acts as a priming kinase for CKI δ/ε.
We previously identified five major in vivo WC-1 phosphorylation sites, located immediately downstream from its DNA-binding domain (He et al. 2005b). The mutation of a single phosphorylation site led to short-period rhythm whereas the mutation of all five sites resulted in arrhythmic phenotype, suggesting that the phosphorylation of multiple sites is critical for the negative feedback process. However, it is not clear whether their phosphorylation is dependent on FRQ. In the study described here, using a quantitative mass spectrometry (MS) method, we demonstrated that these WC-1 sites are phosphorylated sequentially, first by a FRQ-independent kinase then by the FRQ-dependent kinase(s). Our results suggest that protein kinase A (PKA) acts as a FRQ-independent WC kinase and serves as a priming kinase for the casein kinases in WC phosphorylation. Knockout of a PKA catalytic subunit (pkac-1) or the down-regulation of its regulatory subunit abolished normal circadian rhythmicities. In addition to its role in controlling WC phosphorylation and stability, PKA also phosphorylates FRQ. In contrast to the role of casein kinases in promoting FRQ degradation, PKA stabilizes FRQ in a manner similar to the stabilization of hPER2 due to phosphorylation at the FASPS site. Together, these results demonstrate that PKA is a critical component of the Neurospora clock that regulates several processes in the circadian negative feedback loop.
Results
Sequential phosphorylation of WC-1 by FRQ-independent and FRQ-dependent kinases
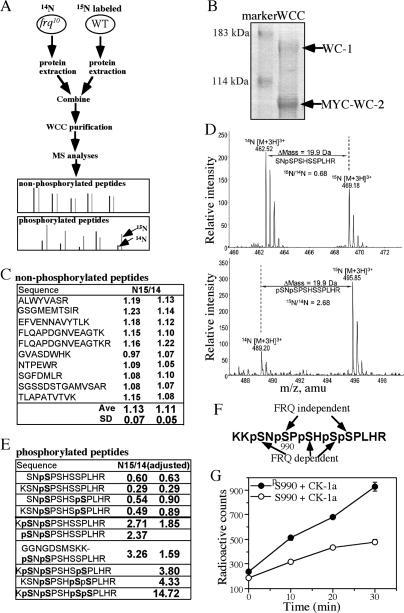
The five WC-1 serine phosphorylation sites near its DNA-binding domain play a critical role in the circadian negative feedback loop (He et al. 2005b). Although CK-1a and CKII have been shown to mediate FRQ-dependent WC phosphorylation, these residues do not resemble typical CKI or CKII phosphorylation sites. However, if one of these sites is first phosphorylated, the rest of the sites will become potential CKI or CKII sites. To understand the regulation of these WC-1 phosphorylation events, we performed quantitative MS experiments (Fig. 1A). It is difficult to use conventional MS methods for quantitative analysis due to experimental variations in different MS runs and varied sensitivity of MS instruments to different peptides. Recently, metabolic stable isotope labeling in combination with MS has been shown to be a powerful and sensitive method for quantitative proteomic analyses. These labeling methods include whole-cell protein labeling in 15N-enriched media (Oda et al. 1999; Washburn et al. 2002). For isotope labeling, cells are cultured in growth medium lacking one essential element but supplemented with its stable isotope-labeled form. In the commonly used Neurospora Vogel’s medium, >99% of nitrogen source comes from NH4NO3, which can be replaced by NH4Cl. We found that circadian conidiation rhythms and WC expression levels were normal when NH4Cl was used as the sole nitrogen source (data not shown).
Figure 1.
The five WC-1 serine residues near its DNA-binding domain are sequentially phosphorylated by FRQ-independent and FRQ-dependent kinases. (A) A diagram depicting the experimental procedures for the quantitative MS analysis of WC-1 phosphorylation using 15N stable isotope labeling of cultures. Both strains contain the Myc-His-WC-2 construct. The bottom panels show hypothetical MS results of the trypsin-digested nonphosphorylated and phosphorylated peptides. (B) Colloidal blue-stained SDS-PAGE gel showing the purified WC complex. (C) A table showing the 15N/14N ratios of 10 typical WC-1 nonphosphorylated peptides from two independent experiments. (D) MS spectra of two of the WC-1 phospho-peptides. Both peptides were triply charged as shown. The m/z of the 15N/14N phospho-peptide pair and their mass difference (Δmass) are indicated. The ratios of the 15N/14N peptide pair were calculated as described in Materials and Methods. (E) A table showing the 15N/14N ratios of the WC-1 phosphorylated peptides covering amino acids 988–995 from two independent experiments. The ratios were adjusted by the relative WC-1 levels in the two strains. (F) A summary of the MS results showing the FRQ-independent and FRQ-dependent WC-1 phosphorylation sites. (G) In vitro phosphorylation of two WC-1 peptides (amino acids 984–1000) by CK-1a. The error bars indicate the standard deviations.
To compare WC phosphorylation profiles between a wild-type strain and a frq-null (frq10) strain, we grew the wc-2ko,Myc-His-WC-2 strain (phenotypically similar to as wild-type strain; He et al. 2002) in medium with 15NH4Cl as the sole nitrogen source, while the frq10,Myc-His-WC-2 strain was cultured in the non-isotope-containing medium (14NH4Cl). The cultures were harvested at DD24 (when WC is extensively phosphorylated in a wild-type strain) and protein extracts were prepared separately. The protein extracts of these strains were mixed at a ratio of 1:2 because of low WC levels in the frq-null strain. The 15N/14N protein extract mixture was then used for WCC purification using our previously established protocol (Fig. 1B; He et al. 2002, 2005b) and the purified 15N/14N WC-1 protein mixture was analyzed by MS after trypsin digestion.
Because all proteins in these two strains were differentially labeled with 15N or normal 14N (15N and 14N differ by 1 Da in molecular weight), on the mass spectrometer, each 15N-labeled peptide has a defined m/z (mass-to-charge ratio) shift relative to its nonlabeled counterpart. The ratio of the relative intensity of each 15N/14N peptide pair on mass spectra directly represents the ratio of their abundances (Fig. 1A). Because the labeled and nonlabeled proteins are processed and analyzed together as one sample, experimental variations due to separate protein purification and sample processing were eliminated. Therefore, this method provides accurate quantitative measurements of protein phosphorylation levels for phospho-peptides. For a FRQ-dependent WC-1 phospho-peptide, its 15N/14N ratio should be >1 if the 15N-labeled and unlabeled WC-1 proteins are present in equal amount.
The nonphosphorylated peptides of WC-1 were used as the internal controls to determine the relative WC-1 levels from these two strains. Figure 1C shows the 15N/14N ratios of 10 typical WC-1 nonphosphorylated peptides from two independent experiments. The results indicated that the levels of WC-1 from the two strains were about the same; the average 15N/14N ratio was ∼1.1 with standard deviations for the two experiments of 0.05 and 0.07, respectively, indicating the accuracy of the method.
There was a total of 10 different WC-1 phospho-peptides covering the region of amino acids 988–999 detected in these two experiments, with one to four phosphorylated serines. Figure 1D shows the representative MS profiles for two of the phospho-peptides in one of the experiments. The 15N/14N ratios (after adjusting for WC-1 protein levels from the two strains) for all phospho-peptides are shown in Figure 1E. Among all of these phospho-peptides, S990 was ubiquitously phosphorylated and was the only site that could be singly phosphorylated. These results suggest that the phosphorylation at these five sites occurs sequentially and that S990 is phosphorylated prior to the phosphorylation of other sites. In addition, the peptides with S990 singly phosphorylated or S990 and S995 doubly phosphorylated showed significantly lower 15N/14N ratios (from 0.29 to 0.90) than the control peptides, indicating that the phosphorylation of these two sites was not FRQ dependent. Furthermore, the low 15N/14N ratios of these peptides indicate that the presence of FRQ in the wild-type strain reduced their levels, probably by promoting the conversion of these phospho-peptides into other peptide species.
Strongly supporting this notion, the 15N/14N ratios of other phospho-peptides that had S988–S990, S988–S990–S995, S990–S994–S995, or S988–S990–S994–S995 phosphorylated were all significantly greater than those of the control peptides. In addition, as the number of phosphorylation sites increased, the 15N/14N ratios also increased significantly. In the case of the phospho-peptide with four sites phosphorylated, the 15N/14N ratio went up to 14.72, indicating that the previous phosphorylation events further promoted the sequential phosphorylation of additional sites. Clearly, the phosphorylation of S988 and S994 is significantly promoted by FRQ and the phosphorylation at these sites is dependent on the phosphorylation of S990. Consistent with this conclusion, we also did not find any S988, S992, or S994 singly phosphorylated peptides in separate nonquantitative MS experiments. Although the phospho-peptide with S992 phosphorylated was identified in our previous experiments, it was not found by MS in these two 15N experiments.
Taken together, these MS results indicate that the phosphorylation of WC-1 at these five sites occurs sequentially (Fig. 1F). The S990 site is first phosphorylated and the phosphorylations of S990 and S995 are independent of FRQ. Afterward, FRQ promotes the phosphorylation of the other three sites. These results suggest that, although none of these five sites resemble good CKI or CKII phosphorylation sites—CKI and CKII prefer acidic residues upstream of or downstream from the target site, respectively—the phosphorylation of S990 can convert the rest of the serine residues into efficient CKI or CKII sites.
To directly test this hypothesis, we phosphorylated two WC-1 synthetic peptides (amino acids 984–1000; S990 is either phosphorylated [pS990] or nonphosphorylated [S990]) by the purified recombinant Neurospora CK-1a in vitro. As shown in Figure 1G, although CK-1a could modestly phosphorylate the nonphosphorylated S990 peptide, the phosphorylation of S990 significantly enhanced its ability to phosphorylate the pS990 peptide. This result suggests that the phosphorylation S990 is a priming event for the efficient phosphorylation of this region by the casein kinases.
PKAC-1 is required for most of the PKA activity in Neurospora
Since CK-1a and CKII mediate the FRQ-dependent WC phosphorylation, the phosphorylation of S990 of WC-1 should be mediated by another kinase. S990 of WC-1 is downstream from two lysine residues and is predicted to be a potential PKA phosphorylation site (http://mendel.imp.ac.at/sat/pkaPS), raising the possibility that PKA is a priming kinase for WC phosphorylation. The eukaryotic PKA holoenzyme consists of catalytic subunits and inhibitory regulatory subunits. The binding of cyclic AMP (cAMP) to the regulatory subunit results in its dissociation from the holoenzyme and the activation of the catalytic subunit (Taylor et al. 2005). In Neurospora, the level of cAMP shows a daily rhythm (Hasunuma et al. 1987), and inhibitors of cAMP phosphodiesterase lengthen the period of the circadian conidiation rhythm (Feldman 1975). In addition, PKA is known to regulate Neurospora growth and development (Bruno et al. 1996; Banno et al. 2005).
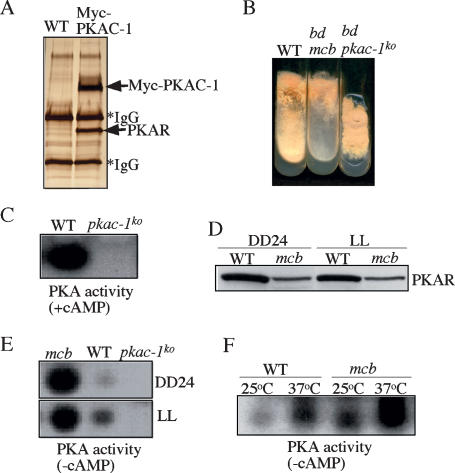
In Neurospora, two genes, pkac-1 and pkac-2, encode the PKA catalytic subunits, and PKAC-1 is the major catalytic subunit (Banno et al. 2005). To understand the molecular components of the PKA holoenzyme in Neurospora, we created a Neurospora strain (Myc-PKAC-1) in which PKAC-1 is tagged by a 6-His and a 5-c-Myc tag (He et al. 2005a). The purification of Myc-His-PKAC-1 from this strain and protein identification by MS revealed only one regulatory subunit protein (named PKAR, for PKA regulatory subunit) in the PKA holoenzyme (Fig. 2A).
Figure 2.
Characterization of the Neurospora PKA mutants. (A) A silver-stained SDS-PAGE gel showing the purified PKA holoenzyme from the Myc-PKAC-1 strain. A wild-type strain that lacks the Myc-tagged construct was used as the negative control. The indicated PKA subunits were confirmed by MS-based protein sequencing. (B) A photograph of the indicated Neurospora strains grown in minimal medium slants. (C) PKA assay in the presence of cAMP showing the lack of detectable PKA activity in the pkac-1ko strain. (D) Western blot analysis showing the low levels of PKAR protein in the mcb strain. Cultures were grown in LL or DD at room temperature. (E) PKA assay (without cAMP) showing the significantly higher PKA activity in the mcb strain than that in the wild-type strain. (F) PKA assay (without cAMP) showing that high temperature led to a significant increase of PKA activity in both the wild-type and mcb strains.
To understand the role of PKA in the Neurospora circadian clock, we created a knockout strain for pkac-1 by gene replacement. As seen in Figure 2B, although PKAC-1 is not essential for survival, the pkac-1ko strain exhibited severe growth and developmental defects, producing few aerial hyphae and conidia. A PKA activity assay showed that the mutant strain had lost all detectable PKA activity (Fig. 2C), indicating that PKAC-1 contributes most of the PKA activity in Neurospora.
mcb (microcycle blastoconidiation) is a pkar mutant with reduced PKAR expression and increased PKA activity
The activity of the catalytic subunit of PKA is negatively inhibited by the regulatory subunit. We were not able to generate a homokaryotic pkar knockout strain, although the knockout strain can be maintained in heterokaryon (data not shown), suggesting that pkar is an essential gene in Neurospora. mcb, a previously isolated temperature-sensitive Neurospora mutant, was thought to be due to a mutation of pkar (Bruno et al. 1996), but the nature and effect of the mutation are not known. When we sequenced the PKAR ORF in the mcb mutant, we found no mutation (data not shown), but a 19-base-pair (bp) (nucleotides 289–307 upstream of the ORF) deletion was identified in the putative 5′ untranslated region (UTR) of the gene. Examination of the pkar mRNA revealed no significant difference between the wild-type and mcb strains, suggesting that the deletion in the mcb mutant did not affect the pkar transcription. However, Western blot analysis using a PKAR-specific antibody showed that PKAR protein levels were significantly reduced (∼20% of the wild-type level) in the mcb mutant (Fig. 2D). The reduction of PKAR expression should result in increased PKA activity. As expected, compared with the wild-type strain, the PKA activity was significantly increased in the mcb mutant (Fig. 2E).
At room temperature, the growth of mcb mutant was near normal, albeit with significantly reduced conidial production (Fig. 2B). At restrictive temperature (37°C), the mcb strain fails to grow due to loss of growth polarity (Bruno et al. 1996). To understand the molecular basis of the temperature-sensitive phenotype of the mcb mutant, we compared the PKA activity in the wild-type and mcb strains grown at different temperatures. As shown in Figure 2F, PKA activity was significantly increased in both strains at 37°C compared with room temperature, but at both temperatures, PKA activity was significantly higher in the mcb strain than that in the wild-type strain. Since the knockout of pkar is lethal in Neurospora, a very high PKA activity in the mcb strain at 37°C should arrest cell growth. Thus, the temperature sensitivity of the mcb mutant should be due to temperature-dependent increase of PKA activity.
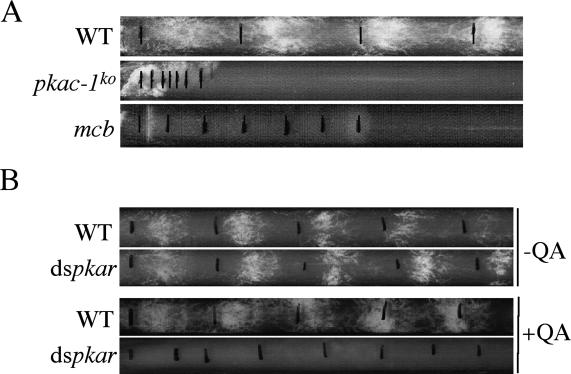
PKA subunits are required for circadian clock function
To understand the role of PKA in the circadian clock, we first examined circadian conidiation rhythm of the mutant strains at room temperature by race tube assay. As shown in Figure 3A, both the pkac-1ko and mcb strains grew more slowly than the wild-type strain and no circadian conidiation rhythms were observed in either mutant strain. To confirm that the mcb phenotype is indeed due to the reduction of PKAR expression, we created a wild-type strain (dspkar) that carries a construct that allows inducible expression of a double-stranded RNA (dsRNA) specific for pkar. The pkar dsRNA construct is under the control of a quinic acid (QA)-inducible promoter so that the pkar expression is silenced by the addition of the inducer QA (Cheng et al. 2005; Maiti et al. 2007). As shown in Figure 3B, in the absence of QA, both the wild-type and dspkar strains exhibited robust circadian conidiation rhythms. However, in the presence of QA, the rhythms in the wild-type strain persisted, but the dspkar strain exhibited no circadian conidiation rhythm. These results indicate that both the loss of PKAC-1 and increase of PKA activity abolish the overt circadian rhythmicity in Neurospora.
Figure 3.
Circadian conidiation rhythms are abolished in PKA mutants. (A) Race tube assay of the wild-type, pkac-1ko, and mcb strains. The daily growth fronts of the cultures are marked by black lines. (B) Race tube assay of the wild-type and dspkar strains in the absence or presence of QA (0.01 M).
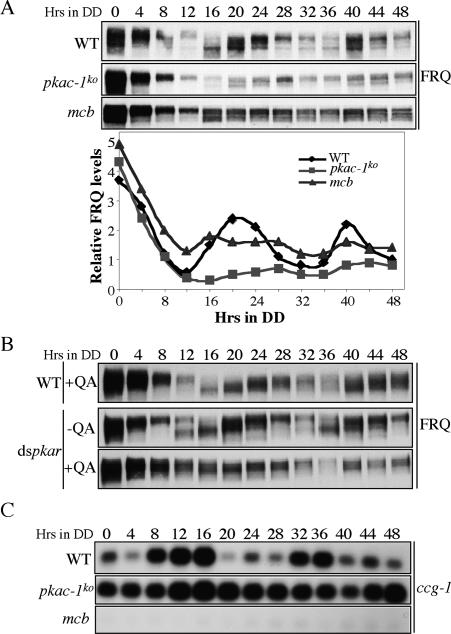
We then investigated the circadian rhythms at the molecular level. As shown in Figure 4A, while there were robust oscillations of FRQ amounts and its phosphorylation status in DD in the wild-type strain, such robust oscillations were abolished in both the pkac-1ko and mcb strains after the initial light-to-dark (LD) transition. In the pkac-1ko strain, the FRQ levels were comparable with that in the wild-type strain in constant light (LL, time 0); however, the levels in DD were significantly lower than those in the wild-type strain and might fluctuate with a very low amplitude. On the other hand, the FRQ levels and its phosphorylation profile stayed constant at intermediate levels in the mcb strain in DD. Consistent with this, the FRQ oscillations were also largely abolished in the dspkar strain in the presence of QA (Fig. 4B; Supplementary Fig. 1A). These results suggest that the proper control of PKA activity is essential for robust oscillations of FRQ and that PKA regulates FRQ levels.
Figure 4.
The normal circadian rhythms at the molecular levels are abolished in the PKA mutants. (A) Western blot analysis of the indicated strains showing the expression of FRQ in DD at different time points. The densitometric results of Western blots are shown in the bottom panel. Similar results were obtained in three independent experiments. (B) Western blot analyses showing the loss of FRQ oscillation in the dspkar strain when pkar was inducibly silenced (QA concentration was 0.01 M). (C) Northern blot analyses showing the expression of ccg-1.
Consistent with these results, we also found that the clock-controlled gene 1 (ccg-1) mRNA rhythm was abolished in both the pkac-1ko and mcb strains, although these mutants exhibited opposite phenotypes (Fig. 4C; Supplementary Fig. 1B). In the pkac-1ko strain, ccg-1 levels were constantly high, whereas in the mcb strain ccg-1 levels were constantly low. Taken together, these data indicate that both of the PKA subunits are required for the normal function of the Neurospora circadian clock.
PKA stabilizes WC-1 and WC-2
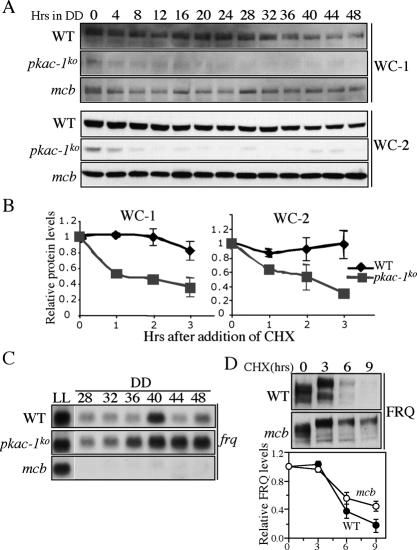
The low FRQ levels in the pkac-1ko strain prompted us to examine the expression of WCs in the pkac-1ko and mcb strains. As shown in Figure 5A, the levels of both WC-1 and WC-2 in DD and LL were significantly lower in the pkac-1ko strain than those in the wild-type strain. The differences in WC levels were especially dramatic in DD: After the LD transition, both WC-1 and WC-2 levels were further decreased in DD in the pkac-1ko strain. On the other hand, the WC-1 and WC-2 levels in mcb strain were comparable with those in the wild-type strain.
Figure 5.
PKA stabilizes WCs and FRQ and represses frq transcription. (A) Western blot analyses of WC-1 and WC-2 showing the low WC-1 and WC-2 levels in the pkac-1ko strain. Cultures were harvested at the indicated DD time points. The experiments for three strains were performed at the same time. Similar results were obtained in three independent experiments. (B) WC-1 and WC-2 are not stable in the pkac-1ko strain. Protein stability was determined by measuring WC levels after the addition of CHX (10 μg/mL). The summary of three independent experiments is shown. (C) Northern blot analysis showing the frq mRNA levels in the indicated strains at different time points in LL and DD. (D) Western blot analysis showing that FRQ is more stable in the mcb strain than the wild-type strain. Densitometric analyses of the results from four independent experiments are shown below.
The low WC levels in the pkac-1ko strain suggest that WCs are unstable in the absence of PKA. To test this possibility, we compared the WC stability in the wild-type and pkac-1ko strains after the addition of the protein synthesis inhibitor cycloheximide (CHX). As shown in Figure 5B, both WC-1 and WC-2 degraded significantly faster in the pkac-1ko strain than in the wild-type strain. These results indicate that PKA stabilizes the WC proteins.
PKA inhibits frq transcription but stabilizes FRQ protein
If WCC activity is similar in the wild-type and pkac-1ko strains, we should expect to see low frq mRNA levels in the pkac-1ko strain. On the contrary, we found that the levels of frq mRNA in the pkac-1ko strain were comparable with or higher than the wild-type levels in DD (Fig. 5C). In contrast, the frq mRNA levels were drastically reduced in the mcb strain in DD despite its near normal amounts of WC proteins. Interestingly, in LL, the frq mRNA levels were comparable in the wild-type and mutant strains, suggesting that effects of PKA on frq transcription are mostly limited to DD. These data suggest that PKA strongly inhibits frq transcription in DD.
The intermediate FRQ protein levels but extremely low frq mRNA levels in DD in the mcb strain (Figs. 4A, 5C) suggest that FRQ is more stable in this mutant. Further supporting this conclusion, the levels of FRQ were low in the pkac-1ko strain despite the high frq mRNA levels (Figs. 4A, 5C). When we examined FRQ stability in DD (DD20), as expected, we found that FRQ degradation rate was significantly slower in the mcb strain than in the wild-type strain (Fig. 5D). These results indicate that, in contrast to CKI and CKII that promote FRQ degradation, PKA stabilizes FRQ.
PKA inhibits WCC DNA-binding activity
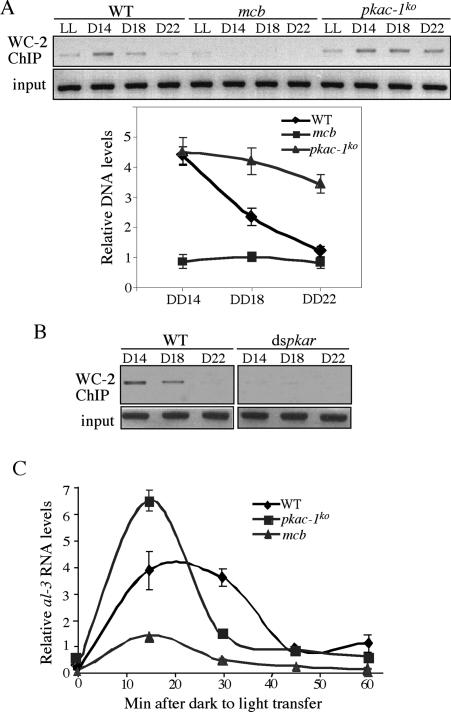
To investigate whether PKA inhibits frq transcription by inhibiting the WCC activity, we performed chromatin immunoprecipitation (ChIP) assays to examine the WCC binding to the C-box of frq promoter in vivo. As shown in Figure 6A, in agreement with our previous results (He et al. 2006), the WCC binding to the C-box was rhythmic in the wild-type strain, peaking at DD14 and low at DD22. Despite the extremely low WC levels in the pkac-1ko strain (Fig. 5A), the WCC binding to the C-box stayed at the wild-type peak levels in DD. In contrast, the WCC-binding levels in the mcb strain were very low (lower than the wild-type trough level) even though its WC levels were comparable with those in the wild-type strain. Consistent with this result, there was also very little WCC binding to the C-box in the dspkar strain when the pkar expression was silenced (Fig. 6B). These results were in agreement with the results of frq mRNA expression in these mutants. Together, they indicate that, like CKI and CKII, PKA inhibits frq transcription by inactivating WCC, suggesting that these kinases act in the same pathway. The strong inhibitory role of PKA on WCC activity suggests that PKA acts upstream of the casein kinases in WCC inhibition.
Figure 6.
PKA inhibits WCC binding to the frq C-box. (A) ChIP assays using WC-2 antibody showing the WCC binding to the frq C-box in the indicated strains at the indicated time points. The densitometric analyses of the ChIP results from three experiments are shown below. (B) ChIP assays using WC-2 antibody showing the WCC binding to the frq C-box in the wild-type and dspkar strains in the presence of QA (0.01 M). (C) Quantitative RT–PCR results showing the light induction of al-3 mRNA in the indicated three strains. Cultures were transferred to LL at DD24.
In addition to their essential role in the circadian negative feedback loop, WCs are also required for all known light responses in Neurospora and WC-1 functions as the photoreceptor (Froehlich et al. 2002; He et al. 2002; He and Liu 2005). Similar to the role of WC phosphorylation in the dark, light-dependent WC phosphorylation can also inhibit WCC activity as a light-activated transcriptional complex (He and Liu 2005). To examine whether PKA also negatively regulates WCC function in the light response, we examined light-induced albino-3 (al-3, a rapidly light-induced gene) expression. We showed previously that light triggers the WCC binding to the al-3 promoter (He and Liu 2005). As shown in Figure 6C, the light induction of al-3 was elevated in the pkac-1ko strain but was significantly blunted in the mcb strain, indicating that PKA also inhibits the light function of WCC.
PKA associates with WCC and can phosphorylate S990 of WC-1
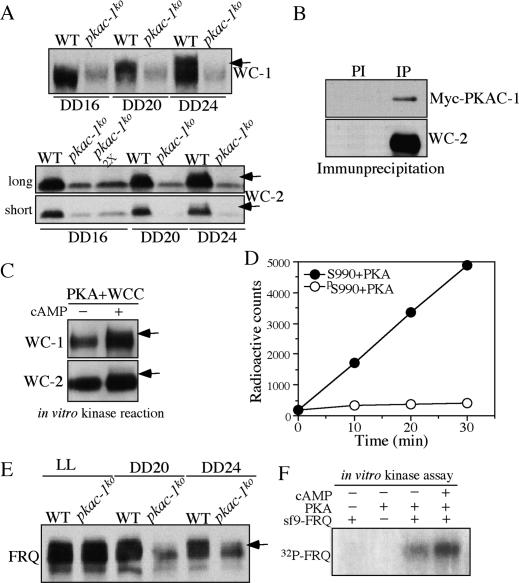
The involvement of a priming kinase for WC phosphorylation and the different substrate preferences of PKA and the casein kinases suggest that PKA may act as a priming kinase for the CK-1a- and CKII-mediated WC phosphorylation. Thus, we compared WC-1 and WC-2 phosphorylation profiles in the wild-type and pkac-1ko strains in DD. As shown in Figure 7A, WC-1 and WC-2 in the wild-type strain were hypophosphorylated at DD16 and became extensively phosphorylated at DD24, similar to previously published results (Schafmeier et al. 2005). In the pkac-1ko strain, however, both WC proteins were hypophosphorylated at these time points, suggesting that PKA regulates WC phosphorylation.
Figure 7.
PKA associates with WCC and phosphorylates WCs and FRQ. (A) Western blot analyses showing the phosphorylation profiles of WC-1 and WC-2 in the wild-type and pkac-1ko strains at the indicated DD time points. Arrows indicate the extensively phosphorylated WC species. (B) Immunoprecipitation (IP) assay showing that WC-2 interacts with Myc-PKAC-1. The Myc-PKAC-1 strain was used. (PI) WC-2 preimmune antiserum was used; (IP) WC-2 antiserum was used. (C) Western blot analysis showing that partially purified PKA phosphorylates WCs in a cAMP-dependent manner. Partially purified PKA was obtained by purifying Myc-PKAC-1 from the Myc-PKAC-1 strain using a nickel column. Equal amounts of proteins were used in both reactions. Arrows indicate the phosphorylated WC species. Note that our WC-1 antibody is more sensitive to phosphorylated WC-1 species than to hypophosphorylated WC-1 (He and Liu 2005). (D) Phosphorylation of WC-1 peptides by PKA. Bovine PKA was used. (E) Western blot analysis showing that FRQ is hypophosphorylated in DD in the pkac-1ko strain. The arrow indicates the extensively phosphorylated FRQ species. (F) In vitro kinase assay showing that purified Neurospora PKA phosphorylates FRQ. PKA was purified from the Myc-PKAC-1 strain by nickel column purification and c-Myc antibody IP.
We then examined whether PKA interacts with WCC in vivo. Using the Myc-PAKC-1 strain, we found that the immunoprecipitation of WC-2 specifically pulled down Myc-PKAC-1, indicating that PKA associates with WCC in vivo (Fig. 7B). On the other hand, immunoprecipitation of FRQ failed to precipitate Myc-PKAC-1 (data not shown), suggesting that the PKA–WCC interaction is independent of FRQ.
To determine whether PKA directly phosphorylates the WCs, we partially purified the Myc-His-PKAC-1 from the pkac-1ko strain using a nickel column. Western blot analyses revealed that PKAC-1, WC-1, and WC-2 were all significantly enriched by the purification, consistent with an association between PKA and WCC (data not shown). We then performed in vitro phosphorylation assay using the purification products in the absence or presence of cAMP. As shown in Figure 7C, significant amounts of WC-1 and WC-2 phosphorylation were observed in the presence of cAMP, suggesting that PKA directly phosphorylates WC proteins. Together, these data suggest that PKA is a WC kinase. However, unlike CK-1a and CKII, which are dependent on FRQ to phosphorylate WCs, PKA’s phosphorylation of WCs is most likely independent of FRQ due to the PKA–WCC association.
We then asked whether PKA can phosphorylate S990 of WC-1 by phosphorylating the WC-1 peptides with commercially available PKA. As shown in Figure 7D, PKA efficiently phosphorylated the S990 peptide but failed to phosphorylate the pS990 peptide, suggesting that S990 is indeed a PKA site. Taken together, these results suggest that PKA and the casein kinases phosphorylate WCs in a sequential manner, with PKA acting as the priming kinase.
FRQ is also a PKA substrate
In addition to PKA’s effects on the WCs, PKA also stabilizes FRQ (Fig. 5D), suggesting that FRQ is also a PKA substrate. To examine this possibility, we compared FRQ phosphorylation profiles in the wild-type and pkac-1ko strains (Fig. 7E). Although the FRQ phosphorylation profiles were very similar in these two strains in LL, at DD20 and DD24, when FRQ in the wild-type strain was extensively phosphorylated, FRQ was mostly hypophosphorylated in the pkac-1ko strain. This result is consistent with our observation that the effect of PKA on FRQ levels is mostly limited to DD (Figs. 4, 5) and suggests that PKA regulates FRQ phosphorylation. To demonstrate that PKA can directly phosphorylate FRQ, we purified Myc-PKA to near homogeneity (as shown in Fig. 2A) from the Myc-PKAC-1 strain. Afterward, the final c-Myc immunoprecipitate was incubated with purified recombinant full-length FRQ (expressed in insect sf9 cells) in the presence of radioactive ATP. As shown in Figure 7F, the purified PKA directly phosphorylated FRQ and the presence of cAMP increased the level of phosphorylation. The significant amount of FRQ phosphorylation without cAMP was most likely due to some of the Myc-PKAC-1 purified without PKAR (Fig. 2A), resulting in cAMP-independent activity. These results suggest that although PKA plays an opposite role in regulating FRQ stability as CK-1a and CKII, it can also phosphorylate FRQ.
Discussion
Phosphorylation of circadian clock proteins is essential for circadian clock mechanisms in both eukaryotes and prokaryotes. In this study, we showed that the phosphorylation sites near the WC-1 DNA-binding domain are phosphorylated in a sequential manner—first by a FRQ-independent kinase, and then by a FRQ-dependent kinase(s), most likely CK-1a or CKII. Our results also suggest that PKA is the kinase that mediates the FRQ-independent WC phosphorylation. Both PKAC-1 and its regulatory subunit PKAR are essential for normal circadian rhythmicity. PKA associates with and can phosphorylate the WC proteins, which leads to their stabilization. Our results indicate that the phosphorylation of WCs by PKA strongly inhibits WCC activity, resulting in the inhibition of frq transcription. In addition to its effects on the WCs, PKA can also phosphorylate FRQ. Importantly, in contrast to the role of CK-1a and CKII in promoting FRQ degradation, phosphorylation by PKA stabilizes FRQ. These data reveal that the PKA-mediated FRQ phosphorylation in Neurospora has effects similar to hPER2 phosphorylation at the FASPS site (Vanselow et al. 2006; Xu et al. 2007). Thus, PKA, in addition to its other cellular functions, is a critical post-translational regulator involved in multiple processes of the Neurospora circadian negative feedback loop.
Sequential WC-1 phosphorylation by FRQ-independent and FRQ-dependent kinases
The five phosphorylation sites immediately downstream from the WC-1 DNA-binding domain are critical for WC-1 function in the circadian negative feedback loop (He et al. 2005b). By performing quantitative MS analyses in combination with whole-cell metabolic stable isotope labeling, we demonstrated that these sites are phosphorylated sequentially (Fig. 1). Serine S990 is the first phosphorylated; S990 and S995 are phosphorylated by a FRQ-independent kinase, and the other sites are then phosphorylated by FRQ-dependent kinase(s). We showed previously that CK-1a and CKII mediate the FRQ-dependent WC phosphorylation to inhibit WCC activity (He et al. 2006). While these five sites do not resemble typical CKI or CKII sites, which are generally upstream of or downstream from acidic residues, the initial phosphorylation of S990 can convert the other sites to efficient CKI or CKII sites. Supporting this notion, we found that the phosphorylation of S990 indeed significantly enhanced the ability of CK-1a to phosphorylate this region (Fig. 1). Thus, our results are consistent with CKI and/or CKII mediation of the FRQ-dependent phosphorylation of WC-1.
The sequential phosphorylation events allow for extensive phosphorylation of WCC, which results in the efficient inhibition of its DNA-binding activity in a FRQ-dependent manner. Consistent with this notion, FRQ-mediated WC phosphorylation and inhibition of frq transcription occur in <2 h (Merrow et al. 1997; Schafmeier et al. 2005, 2006). The phosphorylation of WCs by a priming kinase and the recruitment of CKI and/or CKII to the WCs by FRQ are likely to ensure rapid phosphorylation and inhibition of WCC in a FRQ-dependent manner. It should be noted that in addition to these five WC-1 sites, other WC-1 and WC-2 phosphorylation sites may also contribute to the inhibition of WCC activity. It is not clear whether other WC sites are also regulated in a similar manner.
PKA acts as a priming kinase for CK-1a or CKII in WC phosphorylation
The revelation of FRQ-independent WC-1 phosphorylation indicates the existence of a priming kinase that mediates the WC phosphorylation by CK-1a and CKII. Several lines of evidence presented in this study suggest that PKA plays such a role. First, both WC-1 and WC-2 are hypophosphorylated in the pkac-1ko strain (Fig. 7A). Second, PKA associates with WCC in vivo and can phosphorylate WC-1 and WC-2 (Fig. 7B,C). S990 of WC-1, a priming phoshorylation site of WC-1, can be phosphorylated by PKA. The fact that PKA associates with WCC but not FRQ suggests that the phosphorylation of WCs by PKA is independent of FRQ. Thus, PKA may first phosphorylate S990, and then the other sites are phosphorylated by CK-1a or CKII.
Third, like CK-1a and CKII, PKA inhibits WCC activity and frq transcription. The strong effect of PKA on WCC activity is indicated by the high WCC binding to the C-box and high frq mRNA levels in DD in the pkac-1ko strain despite its extremely low WC protein levels (Figs. 5A,C, 6A). In comparison, the WC levels are higher in the ck-1a mutant and cka mutants than those in the pkac-1ko strain (He et al. 2006). In addition, the increase of PKA activity in the mcb mutant drastically reduces the WCC binding to the frq C-box and inhibits frq transcription to very low levels in DD (Figs. 5C, 6A). These results suggest that PKA acts upstream of CK-1a and CKII in the WCC inhibition process.
Finally, PKA is required for clock function. Both the elimination of most PKA activity by deleting pkac-1 and the increase of PKA activity by reducing its regulatory subunit levels result in the abolishment of robust circadian rhythms at the physiological and molecular levels (Figs. 3, 4). These data suggest that the proper control of PKA activity is critical for clock regulation. Taken together, these data suggest that PKA acts as a priming kinase to mediate FRQ-dependent WCC phosphorylation by CK-1a and CKII. It is also likely, however, that some of the FRQ-dependent WC phosphorylation events (at other sites) can occur independent of PKA, and additional kinase(s) can also contribute to the process. Interestingly, PKA is also known to be a priming kinase for the CKI family members (including DBT) in the Drosophila hedgehog pathway (Jia et al. 2004).
The cAMP level is controlled by the circadian clock in Neurospora (Hasunuma et al. 1987). Thus, although PKA can phosphorylate the WCs independent of FRQ, the daily rhythm of cAMP in a wild-type strain should regulate PKA function rhythmically, forming another post-translational loop in the circadian clock.
The role of PKA in stabilizing FRQ: implications in circadian period determination
Like PER phosphorylation in animals, one of the main functions of FRQ phosphorylation is to promote its degradation through the ubiquitin/proteasome pathway mediated by the ubiquitin E3 ligase SCFFWD-1 (Liu et al. 2000; Gorl et al. 2001; Yang et al. 2002, 2004; He et al. 2003, 2005a, 2006). In this study, we showed that, unlike CK-1a and CKII, which promote FRQ degradation, PKA stabilizes FRQ. In the pkac-1ko strain, the FRQ protein levels are low in DD despite the high frq mRNA levels. In the mcb strain, in which PKA activity is high, frq mRNA levels are extremely low, but FRQ protein is at an intermediate level in DD. In addition, FRQ protein is more stable in the mcb strain than in the wild-type strain. Furthermore, our results suggest that the stabilization of FRQ by PKA is likely due to phosphorylation of FRQ by PKA. Supporting this notion, we found that FRQ is hypophosphorylated in DD in the pkac-1ko strain. In addition, the Neurospora PKA can directly phosphorylate FRQ in vitro. Although PKA does not associate tightly with FRQ, it is possible that, just like FRQ recruits CK-1a and CKII to phosphorylate the WCs, WCC can bring PKA to FRQ due to the association of WCC and PKA.
How does PKA phosphorylation of FRQ inhibit its degradation? In the mammalian WNT signaling pathway, phosphorylation of β-catenin by PKA is known to inhibit the ubiquitination of β-catenin promoted by CKI and GSK-3β phosphorylation (Hino et al. 2005). Thus, it is possible that a similar mechanism is at work here. The FRQ phosphorylation by PKA probably either alters the FRQ structure conformation or inhibits its phosphorylation by CK-1a and CKII, so that it cannot be efficiently ubiquitinated and degraded.
What is the role of these opposite functions of FRQ phosphorylation? It is very likely that the phosphorylation events mediating FRQ degradation or FRQ stabilization occur at different regions on FRQ. These opposite but independent functions of FRQ phosphorylation can maintain the needed precision of the circadian period length, which is largely determined by FRQ stability in this organism.
In addition, the temperature-dependent increase of PKA activity (Fig. 2F) may also contribute to the mechanism of temperature compensation, a fundamental property of all circadian clocks. The temperature-dependent changes in PKA activity may counter potential changes in casein kinase activities at different temperatures, allowing the stability of FRQ to be relatively constant within the physiological temperature range, thus similar period length at different temperatures.
Recent studies of the FASPS mutation site in the human Per2 revealed that the phosphorylation of this site leads to hPer2 stabilization rather than degradation (Vanselow et al. 2006; Xu et al. 2007). The S662G mutation of hPer2 in FASPS abolishes the phosphorylation of downstream sites by CKI δ/ε. S662 is not phosphorylated by CKI, indicating the existence of an unidentified priming kinase. Interestingly, S662 is located downstream from a lysine residue and is predicted to be a potential PKA site. Although the cAMP/PKA pathway has been shown to play an important role in light input of the clock and the output pathways in animals (Prosser and Gillette 1989, 1991; Majercak et al. 1997), the role of PKA in the circadian negative feedback loops is not clear. Because of the conservation of the post-translational regulations of circadian oscillators from Neurospora to mammals, our results raise the possibility that PKA may have similar roles in animal circadian oscillators.
Materials and methods
Strains and culture conditions
87-3 (bd a) was used as the wild-type strain in this study. The bd pkac-1ko strain was created by deleting the entire PKAC-1 ORF through homologous recombination using a protocol described previously (He et al. 2006). A mcb strain (FGSC #7094) was obtained from the Fungal Genetic Stock Center and was crossed with a bd strain to create the bd mcb strain. The dspkar strain was created by introducing a QA-inducible dsRNA construct specific for pkar into a wild-type strain at the his-3 locus as described previously (Cheng et al. 2005). Liquid cultures were grown in minimal medium (1× Vogel’s, 2% glucose). For purification of WCC for the quantitative MS experiments, NH4Cl or 15NH4Cl (Cambridge Isotope Laboratories) was used to replace NH4NO3 in the Vogel’s medium. When QA was used, liquid cultures were grown in 0.01 M QA (pH 5.8), 1× Vogel’s, 0.1% glucose, and 0.17% arginine. Race tube medium contained 1× Vogel’s, 0.1% glucose (0% when QA was used), 0.17% arginine, 50 ng/mL biotin, and 1.5% agar.
PKA assay
PKA assays were performed using the PepTag nonradioactive PKA assay kit (Promega) following the manufacturer’s protocol. The tissue was extracted by an extraction buffer (25 mM Tris-HCl at pH 7.4, 0.5 mM EDTA, 0.5 mM EGTA, 10 mM β-mercaptoethanol, 1 μg/mL leupeptin, 1 μg/mL aprotinin, 1 mM PMSF) and 10 μg of protein were used in each assay. The reactions were kept for 30 min at 30°C. PKA activity was visualized by agarose gel electrophoresis of a fluorescent PKA substrate. The gel was photographed under UV light.
Generation of antiserum against PKAR
The GST-PKAR fusion protein (containing PKAR amino acids 1–378) was expressed in BL21 cells, and the soluble proteins were purified with Glutathione-Uniflow Resin (Clontech) and used as the antigen to generate rabbit polyclonal antiserum (Cocalico Biologicals, Inc.).
Purification of the Myc-His-PKAC-1 from Neurospora
The pkac-1ko, Myc-His-PKAC-1, and wild-type strains (as the negative control) were cultured in LL in liquid medium containing 0.01 M QA, 1× Vogel’s, 0.1% glucose, and 0.17% arginine before harvesting (∼25 g of tissue). The purification was performed as described previously (He et al. 2002, 2005a). The final c-Myc precipitates were separated in SDS-PAGE gel and the gel was subsequently silver-stained according to the manufacturer’s instructions (SiverQuest, Invitrogen). The specific bands were excised and subjected to tryptic digestion and nano-HPLC/electrospray MS (ESI-MS) analysis.
MS analyses
The WC-1 proteins in gel were digested with 10 ng/μL sequencing grade trypsin (Promega) in 50 mM NH4HCO3 (pH 7.8) overnight at 37°C. The resulting peptides were extracted with 5% formic acid/50% acetonitrile and 0.1% formic acid/75% acetonitrile sequentially and concentrated to ∼10 μL.
The extracted peptides were analyzed by a capillary analytical column (50 μm × 10 cm) packed with YMC 5-μm spherical C18 revered phase particles (YMC). To elute peptides from the column, an Agilent 1100 series binary pumps system was used to generate the following HPLC gradient: 0%–5% B in 5 min, 5%–40% B in 25 min, and 40%–100% B in 10 min (A = 0.1 M acetic acid in water, B = 0.1 M acetic acid/70% acetonitrile). The eluted peptides were sprayed directly into a QSTAR XL mass spectrometer (MDS SCIEX) equipped with a nano-ESI ion source. The spectra were acquired in “information-dependent” mode. The main parameters used in the acquisition method were as follows: MS scans were from 400 to 2000 Da; the top three most-abundant peaks in the MS scan were selected for MS/MS scans under “enhance all” mode using low resolution for precursor ion isolation; dynamic exclusion time was 20 sec, accumulation time was 1 sec for all scans, and spray voltage was 2.1 KV.
Database searches were performed on a Mascot server (Matrix Science Ltd.). For each sample, two database searches were carried out: one with 14N amino acids and another with 15N amino acids. For quantitative analysis of the identified peptides, the 14N/15N result files from the Mascot search were merged and imported into the open source software MSQuant (http://msquant.sourceforge.net). The 14N/15N peptide pairs were recognized by MSQuant based on the information from Mascot search results and the calculated difference in their m/z. The ratios of the 15N/14N peptide pairs were calculated based on the summed peak intensities from individual Extracted Ion Chromatography (XIC). The average 15N/14N ratio of the 10 typical nonphosphorylated peptides was used as the correction factor to determine the 15N/14N ratios of the phospho-peptides.
Protein and RNA analyses
Protein extraction, Western blot analysis, and immunoprecipitation assays were performed as described previously (Garceau et al. 1997; Cheng et al. 2001a). Equal amounts of total protein (40 μg) were loaded in each protein lane of SDS-PAGE and, after electrophoresis, proteins were transferred onto PVDF membrane and Western blot analyses were performed. To analyze the phosphorylation profiles of WC-2, 10% SDS-PAGE gels containing a ratio of 149:1 acrylamide/bisacrylamide were used. Otherwise, 7.5% SDS-PAGE gels containing a ratio of 37.5:1 acrylamide/bisacrylamide were used.
RNA extraction and Northern blot analyses were performed as described previously (Aronson et al. 1994). Equal amounts of total RNA (20 μg) were loaded onto agarose gels for electrophoresis, and the gels were blotted and probed with an RNA probe specific for frq.
To compare protein or RNA levels from different strains, the experiments were performed side by side and the protein or RNA samples were transferred to the same membrane for Western or Northern blot analysis.
ChIP assay
The ChIP assay was performed as described previously (He and Liu 2005; He et al. 2006). The immunoprecipitation was performed using our WC-2 antibody. Each experiment was independently performed three times and immunoprecipitation without the WC-2 antibody was used as the negative control.
Purification of insect cell-expressed FRQ proteins
Expression of full-length FRQ in sf9 insect cells was performed according to the protocol provided by the manufacturer (Bac-to-Bac Baculovirus Expression System, Invitrogen). The insect cells were infected by a virus expressing His-FRQ (6-His tag at the N terminus). His-tagged proteins were purified with a nickel column.
In vitro phosphorylation
The WC-1 peptides used for the in vitro phosphorylation were MSKKSNSPSHSSPLHRE and MSKKSNpSPSHSSPLHRE. The in vitro phosphorylation of the peptides (100 μM) by CK-1a (1 ng/μL) or PKA (2.5 U/μL) was preformed as described previously (Xu et al. 2007). Recombinant N-terminal His-tagged full-length CK-1a was expressed in Escherichia coli and purified by a nickel column. The bovine PKA catalytic subunit was purchased from Promega.
For WC phosphorylation by PKA, the partially purified Myc-His-PKAC-1 (also containing WCs) from a nickel column was used. The reaction (2.6 μg of protein) took place in a buffer containing 20 mM Tris-HCl (pH 7.4), 10 mM MgCl2, and 1 mM ATP for 30 min at 30°C with or without cAMP (1 μM). For FRQ phosphorylation, His-FRQ purified from sf9 cells (0.2 μg) was incubated with 4 μL of c-Myc agarose beads bound by Myc-His-PKAC-1 in the presence of 6.7 μM [γ32P]ATP (3000 Ci/mmol). For WC-1 and WC-2, Western blotting was performed to detect the phosphorylation pattern changes. For FRQ, phosphorylation was quantitated by autoradiography.
Note added in proof
Since the submission of this manuscript, a paper (Ziv et al. 2007) has been published online that also describes the mutation of pkar in the Neurospora mcb mutant.
Acknowledgments
We thank Lixin Wang for excellent technical assistance, and Drs. Oded Yarden and Michael Plamann for sharing unpublished results of the mcb mutant. This research was supported by grants from the National Institutes of Health and Welch Foundation to Y.L. Y.L. is the Louise W. Kahn Endowed Scholar in Biomedical Research at University of Texas Southwestern Medical Center.
Footnotes
Supplemental material is available at http://www.genesdev.org.
Article is online at http://www.genesdev.org/cgi/doi/10.1101/gad.1610207
References
- Allada R., Emery P., Takahashi J.S., Rosbash M., Emery P., Takahashi J.S., Rosbash M., Takahashi J.S., Rosbash M., Rosbash M. Stopping time: The genetics of fly and mouse circadian clocks. Annu. Rev. Neurosci. 2001;24:1091–1119. doi: 10.1146/annurev.neuro.24.1.1091. [DOI] [PubMed] [Google Scholar]
- Aronson B., Johnson K., Loros J.J., Dunlap J.C., Johnson K., Loros J.J., Dunlap J.C., Loros J.J., Dunlap J.C., Dunlap J.C. Negative feedback defining a circadian clock: Autoregulation in the clock gene frequency. Science. 1994;263:1578–1584. doi: 10.1126/science.8128244. [DOI] [PubMed] [Google Scholar]
- Banno S., Ochiai N., Noguchi R., Kimura M., Yamaguchi I., Kanzaki S., Murayama T., Fujimura M., Ochiai N., Noguchi R., Kimura M., Yamaguchi I., Kanzaki S., Murayama T., Fujimura M., Noguchi R., Kimura M., Yamaguchi I., Kanzaki S., Murayama T., Fujimura M., Kimura M., Yamaguchi I., Kanzaki S., Murayama T., Fujimura M., Yamaguchi I., Kanzaki S., Murayama T., Fujimura M., Kanzaki S., Murayama T., Fujimura M., Murayama T., Fujimura M., Fujimura M. A catalytic subunit of cyclic AMP-dependent protein kinase, PKAC-1, regulates asexual differentiation in Neurospora crassa. Genes Genet. Syst. 2005;80:25–34. doi: 10.1266/ggs.80.25. [DOI] [PubMed] [Google Scholar]
- Belden W.J., Loros J.J., Dunlap J.C., Loros J.J., Dunlap J.C., Dunlap J.C. Execution of the circadian negative feedback loop in Neurospora requires the ATP-dependent chromatin-remodeling enzyme CLOCKSWITCH. Mol. Cell. 2007;25:587–600. doi: 10.1016/j.molcel.2007.01.010. [DOI] [PubMed] [Google Scholar]
- Bruno K.S., Aramayo R., Minke P.F., Metzenberg R.L., Plamann M., Aramayo R., Minke P.F., Metzenberg R.L., Plamann M., Minke P.F., Metzenberg R.L., Plamann M., Metzenberg R.L., Plamann M., Plamann M. Loss of growth polarity and mislocalization of septa in a Neurospora mutant altered in the regulatory subunit of cAMP-dependent protein kinase. EMBO J. 1996;15:5772–5782. [PMC free article] [PubMed] [Google Scholar]
- Cheng P., Yang Y., Heintzen C., Liu Y., Yang Y., Heintzen C., Liu Y., Heintzen C., Liu Y., Liu Y. Coiled-coil domain mediated FRQ–FRQ interaction is essential for its circadian clock function in Neurospora. EMBO J. 2001a;20:101–108. doi: 10.1093/emboj/20.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng P., Yang Y., Liu Y., Yang Y., Liu Y., Liu Y. Interlocked feedback loops contribute to the robustness of the Neurospora circadian clock. Proc. Natl. Acad. Sci. 2001b;98:7408–7413. doi: 10.1073/pnas.121170298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng P., Yang Y., Gardner K.H., Liu Y., Yang Y., Gardner K.H., Liu Y., Gardner K.H., Liu Y., Liu Y. PAS domain-mediated WC-1/WC-2 interaction is essential for maintaining the steady state level of WC-1 and the function of both proteins in circadian clock and light responses of Neurospora. Mol. Cell. Biol. 2002;22:517–524. doi: 10.1128/MCB.22.2.517-524.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng P., Yang Y., Wang L., He Q., Liu Y., Yang Y., Wang L., He Q., Liu Y., Wang L., He Q., Liu Y., He Q., Liu Y., Liu Y. WHITE COLLAR-1, a multifunctional Neurospora protein involved in the circadian feedback loops, light sensing, and transcription repression of wc-2. J. Biol. Chem. 2003;278:3801–3808. doi: 10.1074/jbc.M209592200. [DOI] [PubMed] [Google Scholar]
- Cheng P., He Q., He Q., Wang L., Liu Y., He Q., He Q., Wang L., Liu Y., He Q., Wang L., Liu Y., Wang L., Liu Y., Liu Y. Regulation of the Neurospora circadian clock by an RNA helicase. Genes & Dev. 2005;19:234–241. doi: 10.1101/gad.1266805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crosthwaite S.K., Dunlap J.C., Loros J.J., Dunlap J.C., Loros J.J., Loros J.J. Neurospora wc-1 and wc-2: Transcription, photoresponses, and the origins of circadian rhythmicity. Science. 1997;276:763–769. doi: 10.1126/science.276.5313.763. [DOI] [PubMed] [Google Scholar]
- Denault D.L., Loros J.J., Dunlap J.C., Loros J.J., Dunlap J.C., Dunlap J.C. WC-2 mediates WC-1–FRQ interaction within the PAS protein-linked circadian feedback loop of Neurospora. EMBO J. 2001;20:109–117. doi: 10.1093/emboj/20.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunlap J.C. Molecular bases for circadian clocks. Cell. 1999;96:271–290. doi: 10.1016/s0092-8674(00)80566-8. [DOI] [PubMed] [Google Scholar]
- Dunlap J.C. Proteins in the Neurospora circadian clockworks. J. Biol. Chem. 2006;281:28489–28493. doi: 10.1074/jbc.R600018200. [DOI] [PubMed] [Google Scholar]
- Feldman J.F. Circadian periodicity a neurospora: Alteration by inhibitors of cyclic AMP phosphodiesterase. Science. 1975;190:789–790. doi: 10.1126/science.173018. [DOI] [PubMed] [Google Scholar]
- Froehlich A.C., Liu Y., Loros J.J., Dunlap J.C., Liu Y., Loros J.J., Dunlap J.C., Loros J.J., Dunlap J.C., Dunlap J.C. White Collar-1, a circadian blue light photoreceptor, binding to the frequency promoter. Science. 2002;297:815–819. doi: 10.1126/science.1073681. [DOI] [PubMed] [Google Scholar]
- Froehlich A.C., Loros J.J., Dunlap J.C., Loros J.J., Dunlap J.C., Dunlap J.C. Rhythmic binding of a WHITE COLLAR-containing complex to the frequency promoter is inhibited by FREQUENCY. Proc. Natl. Acad. Sci. 2003;100:5914–5919. doi: 10.1073/pnas.1030057100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garceau N., Liu Y., Loros J.J., Dunlap J.C., Liu Y., Loros J.J., Dunlap J.C., Loros J.J., Dunlap J.C., Dunlap J.C. Alternative initiation of translation and time-specific phosphorylation yield multiple forms of the essential clock protein FREQUENCY. Cell. 1997;89:469–476. doi: 10.1016/s0092-8674(00)80227-5. [DOI] [PubMed] [Google Scholar]
- Gorl M., Merrow M., Huttner B., Johnson J., Roenneberg T., Brunner M., Merrow M., Huttner B., Johnson J., Roenneberg T., Brunner M., Huttner B., Johnson J., Roenneberg T., Brunner M., Johnson J., Roenneberg T., Brunner M., Roenneberg T., Brunner M., Brunner M. A PEST-like element in FREQUENCY determines the length of the circadian period in Neurospora crassa. EMBO J. 2001;20:7074–7084. doi: 10.1093/emboj/20.24.7074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasunuma K., Funadera K., Shinohara Y., Furnkawa K., Watanabe M., Funadera K., Shinohara Y., Furnkawa K., Watanabe M., Shinohara Y., Furnkawa K., Watanabe M., Furnkawa K., Watanabe M., Watanabe M. Circadian oscillation and light induced changes in the concentration of cyclic nycleotides in Neurospora. Curr. Genet. 1987;12:127–133. [Google Scholar]
- He Q., Liu Y., Liu Y. Molecular mechanism of light responses in Neurospora: From light-induced transcription to photoadaptation. Genes & Dev. 2005;19:2888–2899. doi: 10.1101/gad.1369605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Q., Cheng P., Yang Y., Wang L., Gardner K.H., Liu Y., Cheng P., Yang Y., Wang L., Gardner K.H., Liu Y., Yang Y., Wang L., Gardner K.H., Liu Y., Wang L., Gardner K.H., Liu Y., Gardner K.H., Liu Y., Liu Y. White collar-1, a DNA binding transcription factor and a light sensor. Science. 2002;297:840–843. doi: 10.1126/science.1072795. [DOI] [PubMed] [Google Scholar]
- He Q., Cheng P., Yang Y., He Q., Yu H., Liu Y., Cheng P., Yang Y., He Q., Yu H., Liu Y., Yang Y., He Q., Yu H., Liu Y., He Q., Yu H., Liu Y., Yu H., Liu Y., Liu Y. FWD1-mediated degradation of FREQUENCY in Neurospora establishes a conserved mechanism for circadian clock regulation. EMBO J. 2003;22:4421–4430. doi: 10.1093/emboj/cdg425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Q., Cheng P., He Q., Liu Y., Cheng P., He Q., Liu Y., He Q., Liu Y., Liu Y. The COP9 signalosome regulates the Neurospora circadian clock by controlling the stability of the SCFFWD-1 complex. Genes & Dev. 2005a;19:1518–1531. doi: 10.1101/gad.1322205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Q., Shu H., Cheng P., Chen S., Wang L., Liu Y., Shu H., Cheng P., Chen S., Wang L., Liu Y., Cheng P., Chen S., Wang L., Liu Y., Chen S., Wang L., Liu Y., Wang L., Liu Y., Liu Y. Light-independent phosphorylation of WHITE COLLAR-1 regulates its function in the Neurospora circadian negative feedback loop. J. Biol. Chem. 2005b;280:17526–17532. doi: 10.1074/jbc.M414010200. [DOI] [PubMed] [Google Scholar]
- He Q., Cha J., He Q., Lee H., Yang Y., Liu Y., Cha J., He Q., Lee H., Yang Y., Liu Y., He Q., Lee H., Yang Y., Liu Y., Lee H., Yang Y., Liu Y., Yang Y., Liu Y., Liu Y. CKI and CKII mediate the FREQUENCY-dependent phosphorylation of the WHITE COLLAR complex to close the Neurospora circadian negative feedback loop. Genes & Dev. 2006;20:2552–2565. doi: 10.1101/gad.1463506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heintzen C., Liu Y., Liu Y. The Neurospora crassa circadian clock. Adv. Genet. 2007;58:25–66. doi: 10.1016/S0065-2660(06)58002-2. [DOI] [PubMed] [Google Scholar]
- Hino S., Tanji C., Nakayama K.I., Kikuchi A., Tanji C., Nakayama K.I., Kikuchi A., Nakayama K.I., Kikuchi A., Kikuchi A. Phosphorylation of β-catenin by cyclic AMP-dependent protein kinase stabilizes β-catenin through inhibition of its ubiquitination. Mol. Cell. Biol. 2005;25:9063–9072. doi: 10.1128/MCB.25.20.9063-9072.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia J., Tong C., Wang B., Luo L., Jiang J., Tong C., Wang B., Luo L., Jiang J., Wang B., Luo L., Jiang J., Luo L., Jiang J., Jiang J. Hedgehog signalling activity of Smoothened requires phosphorylation by protein kinase A and casein kinase I. Nature. 2004;432:1045–1050. doi: 10.1038/nature03179. [DOI] [PubMed] [Google Scholar]
- Kim E.Y., Edery I., Edery I. Balance between DBT/CKIε kinase and protein phosphatase activities regulate phosphorylation and stability of Drosophila CLOCK protein. Proc. Natl. Acad. Sci. 2006;103:6178–6183. doi: 10.1073/pnas.0511215103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim E.Y., Ko H.W., Yu W., Hardin P.E., Edery I., Ko H.W., Yu W., Hardin P.E., Edery I., Yu W., Hardin P.E., Edery I., Hardin P.E., Edery I., Edery I. A DOUBLETIME kinase binding domain on the Drosophila PERIOD protein is essential for its hyperphosphorylation, transcriptional repression, and circadian clock function. Mol. Cell. Biol. 2007;27:5014–5028. doi: 10.1128/MCB.02339-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King D.P., Takahashi J.S., Takahashi J.S. Molecular genetics of circadian rhythms in mammals. Annu. Rev. Neurosci. 2000;23:713–742. doi: 10.1146/annurev.neuro.23.1.713. [DOI] [PubMed] [Google Scholar]
- Lin J.M., Kilman V.L., Keegan K., Paddock B., Emery-Le M., Rosbash M., Allada R., Kilman V.L., Keegan K., Paddock B., Emery-Le M., Rosbash M., Allada R., Keegan K., Paddock B., Emery-Le M., Rosbash M., Allada R., Paddock B., Emery-Le M., Rosbash M., Allada R., Emery-Le M., Rosbash M., Allada R., Rosbash M., Allada R., Allada R. A role for casein kinase 2α in the Drosophila circadian clock. Nature. 2002;420:816–820. doi: 10.1038/nature01235. [DOI] [PubMed] [Google Scholar]
- Liu Y. Analysis of posttranslational regulations in the Neurospora circadian clock. Methods Enzymol. 2005;393:379–393. doi: 10.1016/S0076-6879(05)93017-6. [DOI] [PubMed] [Google Scholar]
- Liu Y., Bell-Pedersen D., Bell-Pedersen D. Circadian rhythms in Neurospora crassa and other filamentous fungi. Eukaryot. Cell. 2006;5:1184–1193. doi: 10.1128/EC.00133-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Loros J., Dunlap J.C., Loros J., Dunlap J.C., Dunlap J.C. Phosphorylation of the Neurospora clock protein FREQUENCY determines its degradation rate and strongly influences the period length of the circadian clock. Proc. Natl. Acad. Sci. 2000;97:234–239. doi: 10.1073/pnas.97.1.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loros J.J., Dunlap J.C., Dunlap J.C. Genetic and molecular analysis of circadian rhythms in Neurospora. Annu. Rev. Physiol. 2001;63:757–794. doi: 10.1146/annurev.physiol.63.1.757. [DOI] [PubMed] [Google Scholar]
- Lowrey P.L., Shimomura K., Antoch M.P., Yamazaki S., Zemenides P.D., Ralph M.R., Menaker M., Takahashi J.S., Shimomura K., Antoch M.P., Yamazaki S., Zemenides P.D., Ralph M.R., Menaker M., Takahashi J.S., Antoch M.P., Yamazaki S., Zemenides P.D., Ralph M.R., Menaker M., Takahashi J.S., Yamazaki S., Zemenides P.D., Ralph M.R., Menaker M., Takahashi J.S., Zemenides P.D., Ralph M.R., Menaker M., Takahashi J.S., Ralph M.R., Menaker M., Takahashi J.S., Menaker M., Takahashi J.S., Takahashi J.S. Positional syntenic cloning and functional characterization of the mammalian circadian mutation tau. Science. 2000;288:483–492. doi: 10.1126/science.288.5465.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiti M., Lee H.C., Liu Y., Lee H.C., Liu Y., Liu Y. QIP, a putative exonuclease, interacts with the Neurospora Argonaute protein and facilitates conversion of duplex siRNA into single strands. Genes & Dev. 2007;21:590–600. doi: 10.1101/gad.1497607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majercak J., Kalderon D., Edery I., Kalderon D., Edery I., Edery I. D. melanogaster deficient in protein kinase A manifest behavior-specific arrhythmia but normal clock function. Mol. Cell. Biol. 1997;17:5915–5922. doi: 10.1128/mcb.17.10.5915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrow M., Garceau N., Dunlap J.C., Garceau N., Dunlap J.C., Dunlap J.C. Dissection of a circadian oscillation into discrete domains. Proc. Natl. Acad. Sci. 1997;94:3877–3882. doi: 10.1073/pnas.94.8.3877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrow M., Franchi L., Dragovic Z., Gorl M., Johnson J., Brunner M., Macino G., Roenneberg T., Franchi L., Dragovic Z., Gorl M., Johnson J., Brunner M., Macino G., Roenneberg T., Dragovic Z., Gorl M., Johnson J., Brunner M., Macino G., Roenneberg T., Gorl M., Johnson J., Brunner M., Macino G., Roenneberg T., Johnson J., Brunner M., Macino G., Roenneberg T., Brunner M., Macino G., Roenneberg T., Macino G., Roenneberg T., Roenneberg T. Circadian regulation of the light input pathway in Neurospora crassa. EMBO J. 2001;20:307–315. doi: 10.1093/emboj/20.3.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori T., Williams D.R., Byrne M.O., Qin X., Egli M., McHaourab H.S., Stewart P.L., Johnson C.H., Williams D.R., Byrne M.O., Qin X., Egli M., McHaourab H.S., Stewart P.L., Johnson C.H., Byrne M.O., Qin X., Egli M., McHaourab H.S., Stewart P.L., Johnson C.H., Qin X., Egli M., McHaourab H.S., Stewart P.L., Johnson C.H., Egli M., McHaourab H.S., Stewart P.L., Johnson C.H., McHaourab H.S., Stewart P.L., Johnson C.H., Stewart P.L., Johnson C.H., Johnson C.H. Elucidating the ticking of an in vitro circadian clockwork. PLoS Biol. 2007;5:e93. doi: 10.1371/journal.pbio.0050093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima M., Imai K., Ito H., Nishiwaki T., Murayama Y., Iwasaki H., Oyama T., Kondo T., Imai K., Ito H., Nishiwaki T., Murayama Y., Iwasaki H., Oyama T., Kondo T., Ito H., Nishiwaki T., Murayama Y., Iwasaki H., Oyama T., Kondo T., Nishiwaki T., Murayama Y., Iwasaki H., Oyama T., Kondo T., Murayama Y., Iwasaki H., Oyama T., Kondo T., Iwasaki H., Oyama T., Kondo T., Oyama T., Kondo T., Kondo T. Reconstitution of circadian oscillation of cyanobacterial KaiC phosphorylation in vitro. Science. 2005;308:414–415. doi: 10.1126/science.1108451. [DOI] [PubMed] [Google Scholar]
- Nawathean P., Rosbash M., Rosbash M. The doubletime and CKII kinases collaborate to potentiate Drosophila PER transcriptional repressor activity. Mol. Cell. 2004;13:213–223. doi: 10.1016/s1097-2765(03)00503-3. [DOI] [PubMed] [Google Scholar]
- Oda Y., Huang K., Cross F.R., Cowburn D., Chait B.T., Huang K., Cross F.R., Cowburn D., Chait B.T., Cross F.R., Cowburn D., Chait B.T., Cowburn D., Chait B.T., Chait B.T. Accurate quantitation of protein expression and site-specific phosphorylation. Proc. Natl. Acad. Sci. 1999;96:6591–6596. doi: 10.1073/pnas.96.12.6591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price J.L., Blau J., Rothenfluh A., Adodeely M., Kloss B., Young M.W., Blau J., Rothenfluh A., Adodeely M., Kloss B., Young M.W., Rothenfluh A., Adodeely M., Kloss B., Young M.W., Adodeely M., Kloss B., Young M.W., Kloss B., Young M.W., Young M.W. double-time is a new Drosophila clock gene that regulates PERIOD protein accumulation. Cell. 1998;94:83–95. doi: 10.1016/s0092-8674(00)81224-6. [DOI] [PubMed] [Google Scholar]
- Prosser R.A., Gillette M.U., Gillette M.U. The mammalian circadian clock in the suprachiasmatic nuclei is reset in vitro by cAMP. J. Neurosci. 1989;9:1073–1081. doi: 10.1523/JNEUROSCI.09-03-01073.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prosser R.A., Gillette M.U., Gillette M.U. Cyclic changes in cAMP concentration and phosphodiesterase activity in a mammalian circadian clock studied in vitro. Brain Res. 1991;568:185–192. doi: 10.1016/0006-8993(91)91396-i. [DOI] [PubMed] [Google Scholar]
- Reppert S.M., Weaver D.R., Weaver D.R. Molecular analysis of mammalian circadian rhythms. Annu. Rev. Physiol. 2001;63:647–676. doi: 10.1146/annurev.physiol.63.1.647. [DOI] [PubMed] [Google Scholar]
- Sathyanarayanan S., Zheng X., Xiao R., Sehgal A., Zheng X., Xiao R., Sehgal A., Xiao R., Sehgal A., Sehgal A. Posttranslational regulation of Drosophila PERIOD protein by protein phosphatase 2A. Cell. 2004;116:603–615. doi: 10.1016/s0092-8674(04)00128-x. [DOI] [PubMed] [Google Scholar]
- Schafmeier T., Haase A., Kaldi K., Scholz J., Fuchs M., Brunner M., Haase A., Kaldi K., Scholz J., Fuchs M., Brunner M., Kaldi K., Scholz J., Fuchs M., Brunner M., Scholz J., Fuchs M., Brunner M., Fuchs M., Brunner M., Brunner M. Transcriptional feedback of Neurospora circadian clock gene by phosphorylation-dependent inactivation of its transcription factor. Cell. 2005;122:235–246. doi: 10.1016/j.cell.2005.05.032. [DOI] [PubMed] [Google Scholar]
- Schafmeier T., Kaldi K., Diernfellner A., Mohr C., Brunner M., Kaldi K., Diernfellner A., Mohr C., Brunner M., Diernfellner A., Mohr C., Brunner M., Mohr C., Brunner M., Brunner M. Phosphorylation-dependent maturation of Neurospora circadian clock protein from a nuclear repressor toward a cytoplasmic activator. Genes & Dev. 2006;20:297–306. doi: 10.1101/gad.360906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sehgal A. Molecular biology of circadian rhythms. Wiley; Hoboken, NJ: 2004. [Google Scholar]
- Taylor S.S., Kim C., Vigil D., Haste N.M., Yang J., Wu J., Anand G.S., Kim C., Vigil D., Haste N.M., Yang J., Wu J., Anand G.S., Vigil D., Haste N.M., Yang J., Wu J., Anand G.S., Haste N.M., Yang J., Wu J., Anand G.S., Yang J., Wu J., Anand G.S., Wu J., Anand G.S., Anand G.S. Dynamics of signaling by PKA. Biochim. Biophys. Acta. 2005;1754:25–37. doi: 10.1016/j.bbapap.2005.08.024. [DOI] [PubMed] [Google Scholar]
- Toh K.L., Jones C.R., He Y., Eide E.J., Hinz W.A., Virshup D.M., Ptacek L.J., Fu Y.H., Jones C.R., He Y., Eide E.J., Hinz W.A., Virshup D.M., Ptacek L.J., Fu Y.H., He Y., Eide E.J., Hinz W.A., Virshup D.M., Ptacek L.J., Fu Y.H., Eide E.J., Hinz W.A., Virshup D.M., Ptacek L.J., Fu Y.H., Hinz W.A., Virshup D.M., Ptacek L.J., Fu Y.H., Virshup D.M., Ptacek L.J., Fu Y.H., Ptacek L.J., Fu Y.H., Fu Y.H. An hPer2 phosphorylation site mutation in familial advanced sleep phase syndrome. Science. 2001;291:1040–1043. doi: 10.1126/science.1057499. [DOI] [PubMed] [Google Scholar]
- Vanselow K., Vanselow J.T., Westermark P.O., Reischl S., Maier B., Korte T., Herrmann A., Herzel H., Schlosser A., Kramer A., Vanselow J.T., Westermark P.O., Reischl S., Maier B., Korte T., Herrmann A., Herzel H., Schlosser A., Kramer A., Westermark P.O., Reischl S., Maier B., Korte T., Herrmann A., Herzel H., Schlosser A., Kramer A., Reischl S., Maier B., Korte T., Herrmann A., Herzel H., Schlosser A., Kramer A., Maier B., Korte T., Herrmann A., Herzel H., Schlosser A., Kramer A., Korte T., Herrmann A., Herzel H., Schlosser A., Kramer A., Herrmann A., Herzel H., Schlosser A., Kramer A., Herzel H., Schlosser A., Kramer A., Schlosser A., Kramer A., Kramer A. Differential effects of PER2 phosphorylation: Molecular basis for the human familial advanced sleep phase syndrome (FASPS) Genes & Dev. 2006;20:2660–2672. doi: 10.1101/gad.397006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Washburn M.P., Ulaszek R., Deciu C., Schieltz D.M., Yates J.R., Ulaszek R., Deciu C., Schieltz D.M., Yates J.R., Deciu C., Schieltz D.M., Yates J.R., Schieltz D.M., Yates J.R., Yates J.R. Analysis of quantitative proteomic data generated via multidimensional protein identification technology. Anal. Chem. 2002;74:1650–1657. doi: 10.1021/ac015704l. [DOI] [PubMed] [Google Scholar]
- Xu Y., Padiath Q.S., Shapiro R.E., Jones C.R., Wu S.C., Saigoh N., Saigoh K., Ptacek L.J., Fu Y.H., Padiath Q.S., Shapiro R.E., Jones C.R., Wu S.C., Saigoh N., Saigoh K., Ptacek L.J., Fu Y.H., Shapiro R.E., Jones C.R., Wu S.C., Saigoh N., Saigoh K., Ptacek L.J., Fu Y.H., Jones C.R., Wu S.C., Saigoh N., Saigoh K., Ptacek L.J., Fu Y.H., Wu S.C., Saigoh N., Saigoh K., Ptacek L.J., Fu Y.H., Saigoh N., Saigoh K., Ptacek L.J., Fu Y.H., Saigoh K., Ptacek L.J., Fu Y.H., Ptacek L.J., Fu Y.H., Fu Y.H. Functional consequences of a CKIδ mutation causing familial advanced sleep phase syndrome. Nature. 2005;434:640–644. doi: 10.1038/nature03453. [DOI] [PubMed] [Google Scholar]
- Xu Y., Toh K.L., Jones C.R., Shin J.Y., Fu Y.H., Ptacek L.J., Toh K.L., Jones C.R., Shin J.Y., Fu Y.H., Ptacek L.J., Jones C.R., Shin J.Y., Fu Y.H., Ptacek L.J., Shin J.Y., Fu Y.H., Ptacek L.J., Fu Y.H., Ptacek L.J., Ptacek L.J. Modeling of a human circadian mutation yields insights into clock regulation by PER2. Cell. 2007;128:59–70. doi: 10.1016/j.cell.2006.11.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y., Cheng P., Zhi G., Liu Y., Cheng P., Zhi G., Liu Y., Zhi G., Liu Y., Liu Y. Identification of a calcium/calmodulin-dependent protein kinase that phosphorylates the Neurospora circadian clock protein FREQUENCY. J. Biol. Chem. 2001;276:41064–41072. doi: 10.1074/jbc.M106905200. [DOI] [PubMed] [Google Scholar]
- Yang Y., Cheng P., Liu Y., Cheng P., Liu Y., Liu Y. Regulation of the Neurospora circadian clock by casein kinase II. Genes & Dev. 2002;16:994–1006. doi: 10.1101/gad.965102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y., Cheng P., He Q., Wang L., Liu Y., Cheng P., He Q., Wang L., Liu Y., He Q., Wang L., Liu Y., Wang L., Liu Y., Liu Y. Phosphorylation of FREQUENCY protein by casein kinase II is necessary for the function of the Neurospora circadian clock. Mol. Cell. Biol. 2003;23:6221–6228. doi: 10.1128/MCB.23.17.6221-6228.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y., He Q., Cheng P., Wrage P., Yarden O., Liu Y., He Q., Cheng P., Wrage P., Yarden O., Liu Y., Cheng P., Wrage P., Yarden O., Liu Y., Wrage P., Yarden O., Liu Y., Yarden O., Liu Y., Liu Y. Distinct roles for PP1 and PP2A in the Neurospora circadian clock. Genes & Dev. 2004;18:255–260. doi: 10.1101/gad.1152604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young M.W., Kay S.A., Kay S.A. Time zones: A comparative genetics of circadian clocks. Nat. Rev. Genet. 2001;2:702–715. doi: 10.1038/35088576. [DOI] [PubMed] [Google Scholar]
- Yu W., Zheng H., Houl J.H., Dauwalder B., Hardin P.E., Zheng H., Houl J.H., Dauwalder B., Hardin P.E., Houl J.H., Dauwalder B., Hardin P.E., Dauwalder B., Hardin P.E., Hardin P.E. PER-dependent rhythms in CLK phosphorylation and E-box binding regulate circadian transcription. Genes & Dev. 2006;20:723–733. doi: 10.1101/gad.1404406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziv C., Gorovits R., Yarden O., Gorovits R., Yarden O., Yarden O. Carbon source affects PKA-dependent polarity of Neurospora crassa in a CRE-1-dependent and independent manner. Fung. Genet. Biol. 2007 doi: 10.1016/j.fgb.2007.05.005. [DOI] [PubMed] [Google Scholar]