Abstract
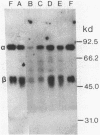
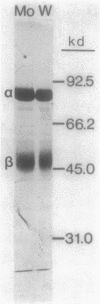
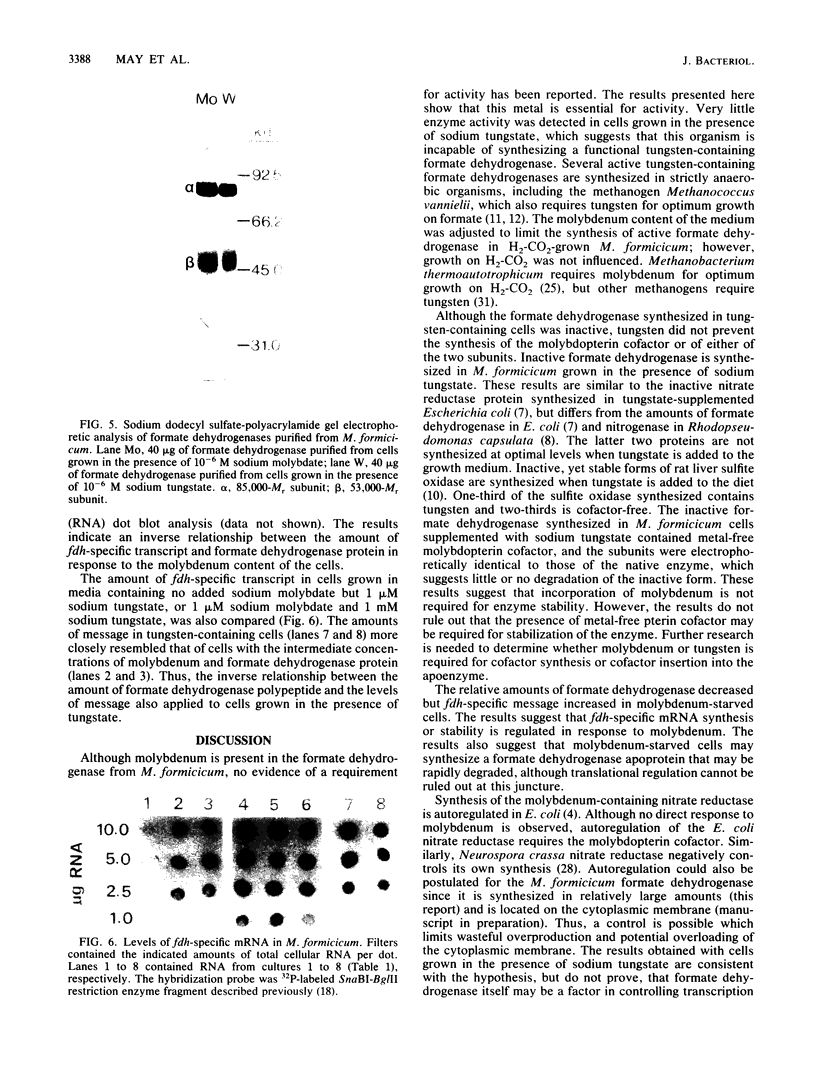
The influence of sodium molybdate and sodium tungstate on formate dehydrogenase activity was studied in H2-CO2-grown cultures of Methanobacterium formicicum. Depletion of molybdate from the growth medium resulted in a 75-fold decrease of intracellular molybdenum and a 35-fold decrease in enzyme activity; however, growth rate and cell yields were not influenced. By using an indirect enzyme-linked immunoassay, the amount of formate dehydrogenase approximated 3% of the total protein in cells grown in the presence of molybdate. Molybdenum-starved cells contained approximately 15-fold less formate dehydrogenase protein; Western blot (immunoblot) analysis revealed that both subunits of the enzyme were synthesized. Molybdenum starvation resulted in an increase in the amount of mRNA that hybridized to fdh-specific DNA. The results indicated an inverse relationship between the amount of transcript and the amount of formate dehydrogenase protein detected in response to molybdenum starvation. The addition of 1 mM tungstate to molybdate-containing media resulted in nearly complete loss of enzyme activity and decreased the intracellular concentration of molybdenum 10-fold. Cells grown in the presence of tungstate synthesized high amounts of inactive formate dehydrogenase and contained mRNA that hybridized to fdh-specific DNA in amounts similar to that in cells grown with sufficient molybdate. Inactive formate dehydrogenase, purified from cells grown in the presence of tungstate, had the same subunit composition and contained amounts of molybdopterin cofactor, albeit metal-free, comparable to those in the active enzyme.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balch W. E., Wolfe R. S. New approach to the cultivation of methanogenic bacteria: 2-mercaptoethanesulfonic acid (HS-CoM)-dependent growth of Methanobacterium ruminantium in a pressureized atmosphere. Appl Environ Microbiol. 1976 Dec;32(6):781–791. doi: 10.1128/aem.32.6.781-791.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber M. J., Siegel L. M., Schauer N. L., May H. D., Ferry J. G. Formate dehydrogenase from Methanobacterium formicicum. Electron paramagnetic resonance spectroscopy of the molybdenum and iron-sulfur centers. J Biol Chem. 1983 Sep 25;258(18):10839–10845. [PubMed] [Google Scholar]
- Bonnefoy V., Pascal M. C., Ratouchniak J., Chippaux M. Autoregulation of the nar operon encoding nitrate reductase in Escherichia coli. Mol Gen Genet. 1986 Jul;204(1):180–184. doi: 10.1007/BF00330207. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Cardenas J., Mortenson L. E. Determination of molybdenum and tungsten in biological materials. Anal Biochem. 1974 Aug;60(2):372–381. doi: 10.1016/0003-2697(74)90244-9. [DOI] [PubMed] [Google Scholar]
- Hallenbeck P. C., Meyer C. M., Vignais P. M. Regulation of nitrogenase in the photosynthetic bacterium Rhodopseudomonas capsulata as studied by two-dimensional gel electrophoresis. J Bacteriol. 1982 Sep;151(3):1612–1616. doi: 10.1128/jb.151.3.1612-1616.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson J. L., Rajagopalan K. V., Cohen H. J. Molecular basis of the biological function of molybdenum. Effect of tungsten on xanthine oxidase and sulfite oxidase in the rat. J Biol Chem. 1974 Feb 10;249(3):859–866. [PubMed] [Google Scholar]
- Johnson J. L., Rajagopalan K. V. Structural and metabolic relationship between the molybdenum cofactor and urothione. Proc Natl Acad Sci U S A. 1982 Nov;79(22):6856–6860. doi: 10.1073/pnas.79.22.6856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones J. B., Stadtman T. C. Methanococcus vannielii: culture and effects of selenium and tungsten on growth. J Bacteriol. 1977 Jun;130(3):1404–1406. doi: 10.1128/jb.130.3.1404-1406.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones J. B., Stadtman T. C. Selenium-dependent and selenium-independent formate dehydrogenases of Methanococcus vannielii. Separation of the two forms and characterization of the purified selenium-independent form. J Biol Chem. 1981 Jan 25;256(2):656–663. [PubMed] [Google Scholar]
- Krol A. J., Hontelez J. G., Roozendaal B., van Kammen A. On the operon structure of the nitrogenase genes of Rhizobium leguminosarum and Azotobacter vinelandii. Nucleic Acids Res. 1982 Jul 24;10(14):4147–4157. doi: 10.1093/nar/10.14.4147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- May H. D., Schauer N. L., Ferry J. G. Molybdopterin cofactor from Methanobacterium formicicum formate dehydrogenase. J Bacteriol. 1986 May;166(2):500–504. doi: 10.1128/jb.166.2.500-504.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller J. B., Scott D. J., Amy N. K. Molybdenum-sensitive transcriptional regulation of the chlD locus of Escherichia coli. J Bacteriol. 1987 May;169(5):1853–1860. doi: 10.1128/jb.169.5.1853-1860.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson M. J., Ferry J. G. Carbon monoxide-dependent methyl coenzyme M methylreductase in acetotrophic Methosarcina spp. J Bacteriol. 1984 Nov;160(2):526–532. doi: 10.1128/jb.160.2.526-532.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel P. S., Ferry J. G. Characterization of the upstream region of the formate dehydrogenase operon of Methanobacterium formicicum. J Bacteriol. 1988 Aug;170(8):3390–3395. doi: 10.1128/jb.170.8.3390-3395.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schauer N. L., Brown D. P., Ferry J. G. Kinetics of Formate Metabolism in Methanobacterium formicicum and Methanospirillum hungatei. Appl Environ Microbiol. 1982 Sep;44(3):549–554. doi: 10.1128/aem.44.3.549-554.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schauer N. L., Ferry J. G. Composition of the coenzyme F420-dependent formate dehydrogenase from Methanobacterium formicicum. J Bacteriol. 1986 Feb;165(2):405–411. doi: 10.1128/jb.165.2.405-411.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schauer N. L., Ferry J. G. FAD requirement for the reduction of coenzyme F420 by formate dehydrogenase from Methanobacterium formicicum. J Bacteriol. 1983 Aug;155(2):467–472. doi: 10.1128/jb.155.2.467-472.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schauer N. L., Ferry J. G., Honek J. F., Orme-Johnson W. H., Walsh C. Mechanistic studies of the coenzyme F420 reducing formate dehydrogenase from Methanobacterium formicicum. Biochemistry. 1986 Nov 4;25(22):7163–7168. doi: 10.1021/bi00370a059. [DOI] [PubMed] [Google Scholar]
- Schauer N. L., Ferry J. G. Metabolism of formate in Methanobacterium formicicum. J Bacteriol. 1980 Jun;142(3):800–807. doi: 10.1128/jb.142.3.800-807.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schauer N. L., Ferry J. G. Properties of formate dehydrogenase in Methanobacterium formicicum. J Bacteriol. 1982 Apr;150(1):1–7. doi: 10.1128/jb.150.1.1-7.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schönheit P., Moll J., Thauer R. K. Nickel, cobalt, and molybdenum requirement for growth of Methanobacterium thermoautotrophicum. Arch Microbiol. 1979 Oct;123(1):105–107. doi: 10.1007/BF00403508. [DOI] [PubMed] [Google Scholar]
- Shuber A. P., Orr E. C., Recny M. A., Schendel P. F., May H. D., Schauer N. L., Ferry J. G. Cloning, expression, and nucleotide sequence of the formate dehydrogenase genes from Methanobacterium formicicum. J Biol Chem. 1986 Oct 5;261(28):12942–12947. [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA transferred or dotted nitrocellulose paper. Methods Enzymol. 1983;100:255–266. doi: 10.1016/0076-6879(83)00060-9. [DOI] [PubMed] [Google Scholar]
- Tomsett A. B., Garrett R. H. Biochemical analysis of mutants defective in nitrate assimilation in Neurospora crassa: evidence for autogenous control by nitrate reductase. Mol Gen Genet. 1981;184(2):183–190. doi: 10.1007/BF00272903. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WOLIN E. A., WOLIN M. J., WOLFE R. S. FORMATION OF METHANE BY BACTERIAL EXTRACTS. J Biol Chem. 1963 Aug;238:2882–2886. [PubMed] [Google Scholar]