Abstract
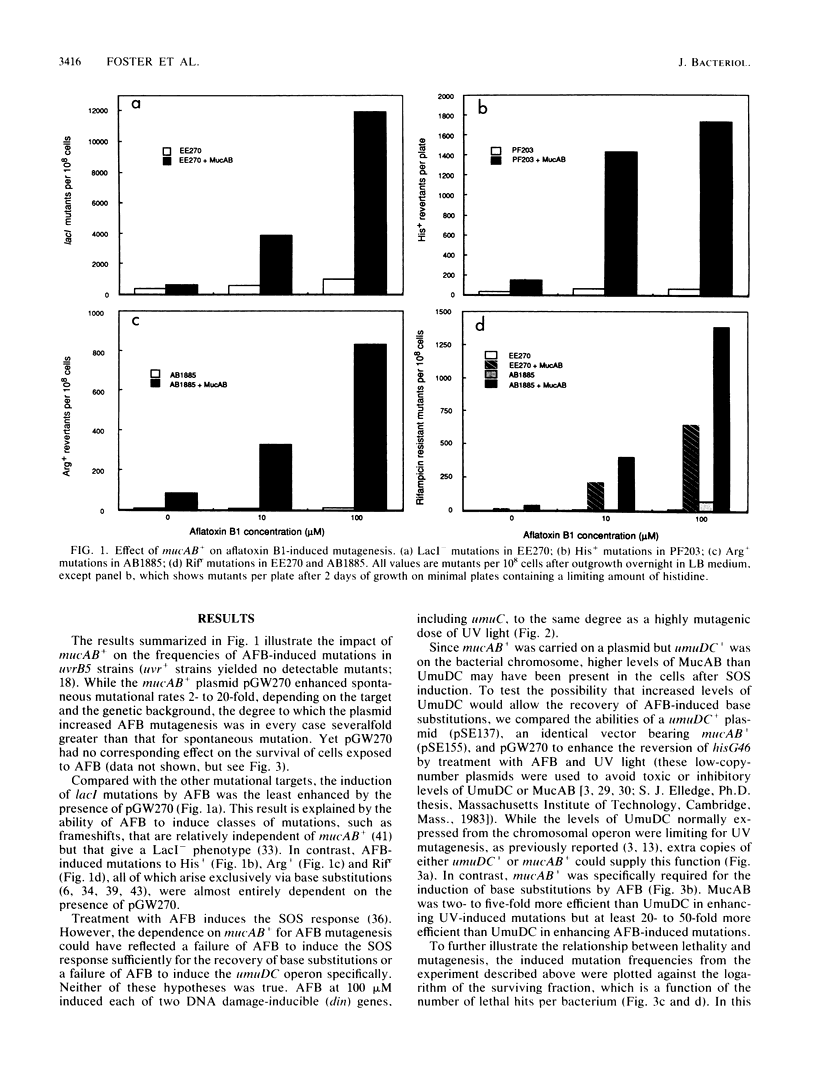
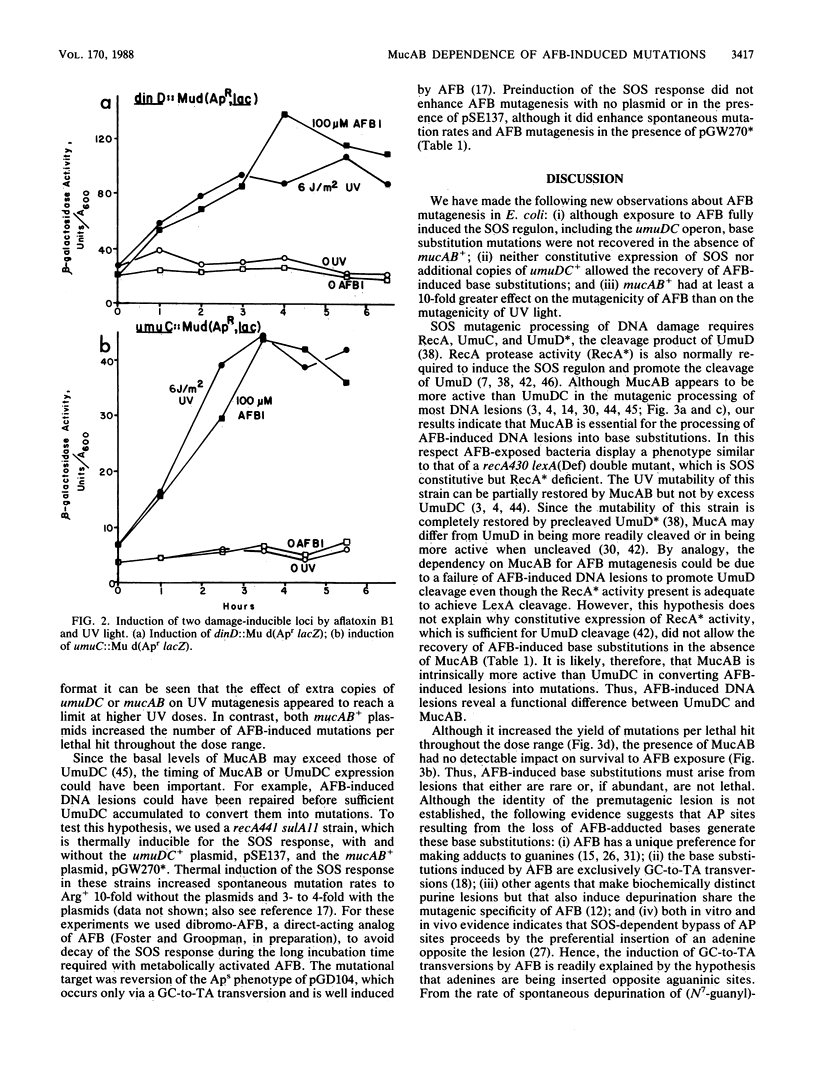
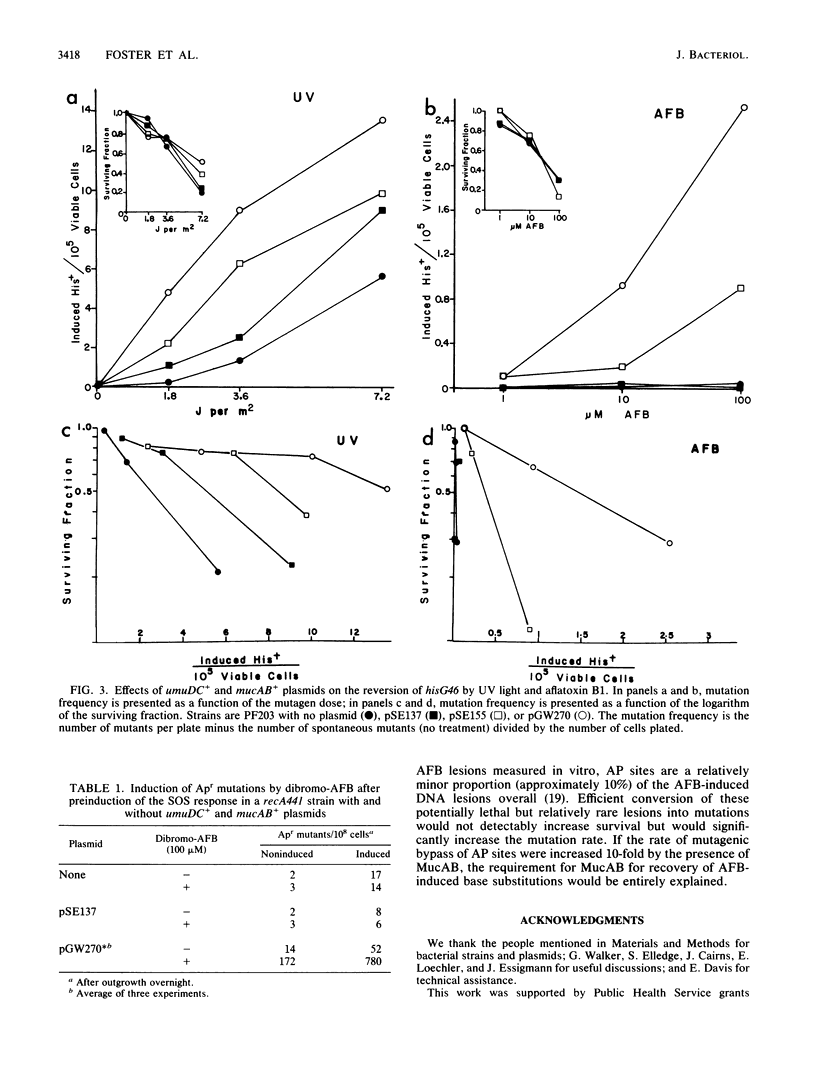
Recovery of aflatoxin B1-induced base substitution mutations in Escherichia coli was almost completely dependent on the presence of the SOS-mutagenesis-enhancing operon mucAB+; the normal E. coli analog, umuDC+, was not sufficient. Yet aflatoxin B1 induced the SOS response, including the umuDC operon, as well as did UV light. Neither preinduction of the SOS response nor the presence of additional copies of umuDC+ allowed the recovery of aflatoxin B1-induced base substitutions. Thus, the premutagenic DNA lesions induced by aflatoxin B1 reveal a functional difference between UmuDC and MucAB. We estimate that in the presence of MucAB the probability that aflatoxin B1-induced DNA lesions will be converted into mutations is increased at least 10-fold.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bagg A., Kenyon C. J., Walker G. C. Inducibility of a gene product required for UV and chemical mutagenesis in Escherichia coli. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5749–5753. doi: 10.1073/pnas.78.9.5749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett R. A., Essigmann J. M., Wogan G. N. Excretion of an aflatoxin-guanine adduct in the urine of aflatoxin B1-treated rats. Cancer Res. 1981 Feb;41(2):650–654. [PubMed] [Google Scholar]
- Blanco M., Herrera G., Aleixandre V. Different efficiency of UmuDC and MucAB proteins in UV light induced mutagenesis in Escherichia coli. Mol Gen Genet. 1986 Nov;205(2):234–239. doi: 10.1007/BF00430433. [DOI] [PubMed] [Google Scholar]
- Blanco M., Herrera G., Collado P., Rebollo J. E., Botella L. M. Influence of RecA protein on induced mutagenesis. Biochimie. 1982 Aug-Sep;64(8-9):633–636. doi: 10.1016/s0300-9084(82)80102-8. [DOI] [PubMed] [Google Scholar]
- Bridges B. A., Woodgate R. Mutagenic repair in Escherichia coli: products of the recA gene and of the umuD and umuC genes act at different steps in UV-induced mutagenesis. Proc Natl Acad Sci U S A. 1985 Jun;82(12):4193–4197. doi: 10.1073/pnas.82.12.4193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burckhardt S. E., Woodgate R., Scheuermann R. H., Echols H. UmuD mutagenesis protein of Escherichia coli: overproduction, purification, and cleavage by RecA. Proc Natl Acad Sci U S A. 1988 Mar;85(6):1811–1815. doi: 10.1073/pnas.85.6.1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess R. R. RNA polymerase. Annu Rev Biochem. 1971;40:711–740. doi: 10.1146/annurev.bi.40.070171.003431. [DOI] [PubMed] [Google Scholar]
- Castellazzi M., George J., Buttin G. Prophage induction and cell division in E. coli. I. Further characterization of the thermosensitive mutation tif-1 whose expression mimics the effect of UV irradiation. Mol Gen Genet. 1972;119(2):139–152. doi: 10.1007/BF00269133. [DOI] [PubMed] [Google Scholar]
- Croy R. G., Wogan G. N. Quantitative comparison of covalent aflatoxin-DNA adducts formed in rat and mouse livers and kidneys. J Natl Cancer Inst. 1981 Apr;66(4):761–768. [PubMed] [Google Scholar]
- Eisenstadt E., Warren A. J., Porter J., Atkins D., Miller J. H. Carcinogenic epoxides of benzo[a]pyrene and cyclopenta[cd]pyrene induce base substitutions via specific transversions. Proc Natl Acad Sci U S A. 1982 Mar;79(6):1945–1949. doi: 10.1073/pnas.79.6.1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elledge S. J., Walker G. C. Proteins required for ultraviolet light and chemical mutagenesis. Identification of the products of the umuC locus of Escherichia coli. J Mol Biol. 1983 Feb 25;164(2):175–192. doi: 10.1016/0022-2836(83)90074-8. [DOI] [PubMed] [Google Scholar]
- Elledge S. J., Walker G. C. The muc genes of pKM101 are induced by DNA damage. J Bacteriol. 1983 Sep;155(3):1306–1315. doi: 10.1128/jb.155.3.1306-1315.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Essigmann J. M., Croy R. G., Nadzan A. M., Busby W. F., Jr, Reinhold V. N., Büchi G., Wogan G. N. Structural identification of the major DNA adduct formed by aflatoxin B1 in vitro. Proc Natl Acad Sci U S A. 1977 May;74(5):1870–1874. doi: 10.1073/pnas.74.5.1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster P. L., Dalbadie-McFarland G., Davis E. F., Schultz S. C., Richards J. H. Creation of a test plasmid for detecting G-C-to-T-A transversions by changing serine to arginine in the active site of beta-lactamase. J Bacteriol. 1987 Jun;169(6):2476–2481. doi: 10.1128/jb.169.6.2476-2481.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster P. L., Davis E. F. Loss of an apurinic/apyrimidinic site endonuclease increases the mutagenicity of N-methyl-N'-nitro-N-nitrosoguanidine to Escherichia coli. Proc Natl Acad Sci U S A. 1987 May;84(9):2891–2895. doi: 10.1073/pnas.84.9.2891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster P. L., Eisenstadt E., Miller J. H. Base substitution mutations induced by metabolically activated aflatoxin B1. Proc Natl Acad Sci U S A. 1983 May;80(9):2695–2698. doi: 10.1073/pnas.80.9.2695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groopman J. D., Croy R. G., Wogan G. N. In vitro reactions of aflatoxin B1-adducted DNA. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5445–5449. doi: 10.1073/pnas.78.9.5445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard-Flanders P., Theriot L. Mutants of Escherichia coli K-12 defective in DNA repair and in genetic recombination. Genetics. 1966 Jun;53(6):1137–1150. doi: 10.1093/genetics/53.6.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaden D. A., Call K. M., Leong P. M., Komives E. A., Thilly W. G. Killing and mutation of human lymphoblast cells by aflatoxin B1: evidence for an inducible repair response. Cancer Res. 1987 Apr 15;47(8):1993–2001. [PubMed] [Google Scholar]
- Kensler T. W., Egner P. A., Davidson N. E., Roebuck B. D., Pikul A., Groopman J. D. Modulation of aflatoxin metabolism, aflatoxin-N7-guanine formation, and hepatic tumorigenesis in rats fed ethoxyquin: role of induction of glutathione S-transferases. Cancer Res. 1986 Aug;46(8):3924–3931. [PubMed] [Google Scholar]
- Kenyon C. J., Walker G. C. DNA-damaging agents stimulate gene expression at specific loci in Escherichia coli. Proc Natl Acad Sci U S A. 1980 May;77(5):2819–2823. doi: 10.1073/pnas.77.5.2819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langer P. J., Shanabruch W. G., Walker G. C. Functional organization of plasmid pKM101. J Bacteriol. 1981 Mar;145(3):1310–1316. doi: 10.1128/jb.145.3.1310-1316.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leadon S. A., Tyrrell R. M., Cerutti P. A. Excision repair of aflatoxin B1-DNA adducts in human fibroblasts. Cancer Res. 1981 Dec;41(12 Pt 1):5125–5129. [PubMed] [Google Scholar]
- Lin J. K., Miller J. A., Miller E. C. 2,3-Dihydro-2-(guan-7-yl)-3-hydroxy-aflatoxin B1, a major acid hydrolysis product of aflatoxin B1-DNA or -ribosomal RNA adducts formed in hepatic microsome-mediated reactions and in rat liver in vivo. Cancer Res. 1977 Dec;37(12):4430–4438. [PubMed] [Google Scholar]
- Loeb L. A. Apurinic sites as mutagenic intermediates. Cell. 1985 Mar;40(3):483–484. doi: 10.1016/0092-8674(85)90191-6. [DOI] [PubMed] [Google Scholar]
- Lu C., Scheuermann R. H., Echols H. Capacity of RecA protein to bind preferentially to UV lesions and inhibit the editing subunit (epsilon) of DNA polymerase III: a possible mechanism for SOS-induced targeted mutagenesis. Proc Natl Acad Sci U S A. 1986 Feb;83(3):619–623. doi: 10.1073/pnas.83.3.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh L., Walker G. C. Cold sensitivity induced by overproduction of UmuDC in Escherichia coli. J Bacteriol. 1985 Apr;162(1):155–161. doi: 10.1128/jb.162.1.155-161.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh L., Walker G. C. New phenotypes associated with mucAB: alteration of a MucA sequence homologous to the LexA cleavage site. J Bacteriol. 1987 May;169(5):1818–1823. doi: 10.1128/jb.169.5.1818-1823.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin C. N., Garner R. C. Aflatoxin B -oxide generated by chemical or enzymic oxidation of aflatoxin B1 causes guanine substitution in nucleic acids. Nature. 1977 Jun 30;267(5614):863–865. doi: 10.1038/267863a0. [DOI] [PubMed] [Google Scholar]
- Miller J. H. Mutagenic specificity of ultraviolet light. J Mol Biol. 1985 Mar 5;182(1):45–65. doi: 10.1016/0022-2836(85)90026-9. [DOI] [PubMed] [Google Scholar]
- Miller J. K., Barnes W. M. Colony probing as an alternative to standard sequencing as a means of direct analysis of chromosomal DNA to determine the spectrum of single-base changes in regions of known sequence. Proc Natl Acad Sci U S A. 1986 Feb;83(4):1026–1030. doi: 10.1073/pnas.83.4.1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller S. S., Eisenstadt E. Suppressible base substitution mutations induced by angelicin (isopsoralen) in the Escherichia coli lacI gene: implications for the mechanism of SOS mutagenesis. J Bacteriol. 1987 Jun;169(6):2724–2729. doi: 10.1128/jb.169.6.2724-2729.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreau P. L., Fanica M., Devoret R. Cleavage of lambda repressor and induction of recA protein synthesis elicited by aflatoxin B1 metabolites in Escherichia coli. Carcinogenesis. 1980;1(10):837–848. doi: 10.1093/carcin/1.10.837. [DOI] [PubMed] [Google Scholar]
- Mortelmans K. E., Stocker B. A. Ultraviolet light protection, enhancement of ultraviolet light mutagenesis, and mutator effect of plasmid R46 in Salmonella typhimurium. J Bacteriol. 1976 Oct;128(1):271–282. doi: 10.1128/jb.128.1.271-282.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nohmi T., Battista J. R., Dodson L. A., Walker G. C. RecA-mediated cleavage activates UmuD for mutagenesis: mechanistic relationship between transcriptional derepression and posttranslational activation. Proc Natl Acad Sci U S A. 1988 Mar;85(6):1816–1820. doi: 10.1073/pnas.85.6.1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry K. L., Elledge S. J., Mitchell B. B., Marsh L., Walker G. C. umuDC and mucAB operons whose products are required for UV light- and chemical-induced mutagenesis: UmuD, MucA, and LexA proteins share homology. Proc Natl Acad Sci U S A. 1985 Jul;82(13):4331–4335. doi: 10.1073/pnas.82.13.4331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Refolo L. M., Bennett C. B., Humayun M. Z. Mechanisms of frameshift mutagenesis by aflatoxin B1-2,3-dichloride. J Mol Biol. 1987 Feb 20;193(4):609–636. doi: 10.1016/0022-2836(87)90344-5. [DOI] [PubMed] [Google Scholar]
- Shinagawa H., Iwasaki H., Kato T., Nakata A. RecA protein-dependent cleavage of UmuD protein and SOS mutagenesis. Proc Natl Acad Sci U S A. 1988 Mar;85(6):1806–1810. doi: 10.1073/pnas.85.6.1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd P. A., Monti-Bragadin C., Glickman B. W. MMS mutagenesis in strains of Escherichia coli carrying the R46 mutagenic enhancing plasmid: phenotypic analysis of Arg+ revertants. Mutat Res. 1979 Sep;62(2):227–237. doi: 10.1016/0027-5107(79)90081-2. [DOI] [PubMed] [Google Scholar]
- Waleh N. S., Stocker B. A. Effect of host lex, recA, recF, and uvrD genotypes on the ultraviolet light-protecting and related properties of plasmid R46 in Escherichia coli. J Bacteriol. 1979 Feb;137(2):830–838. doi: 10.1128/jb.137.2.830-838.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker G. C. Mutagenesis and inducible responses to deoxyribonucleic acid damage in Escherichia coli. Microbiol Rev. 1984 Mar;48(1):60–93. doi: 10.1128/mr.48.1.60-93.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker G. C. Plasmid (pKM101)-mediated enhancement of repair and mutagenesis: dependence on chromosomal genes in Escherichia coli K-12. Mol Gen Genet. 1977 Mar 28;152(1):93–103. doi: 10.1007/BF00264945. [DOI] [PubMed] [Google Scholar]



