Abstract
Transgenic mice containing an upstream glucokinase (βGK) promoter– simian virus 40 T antigen (Tag) fusion gene develop neuroendocrine tumors primarily in the pancreas, gut, and pituitary. Pancreatic tumors from a line with delayed tumorigenesis were of two different types: insulinomas and noninsulinomas. The noninsulinomas are often periductal in location, express none of the four major islet peptide hormones, Glut-2, Pdx1, tyrosine hydroxylase, Pax4, Pax6, or Nkx6.1, but do express glucokinase, Sur1, Isl1, Hnf3β, Hnf6, Beta2/NeuroD, and Nkx2.2. Cells from two different noninsulinoma tumors, when adapted to culture, began to express either insulin, glucagon, or somatostatin. Given the partial gene expression repertoire of the noninsulinoma tumors, their apparent periductal origin, and the ability of these cells to partially cytodifferentiate in culture, we suggest that these tumors are derived from islet progenitor cells. Thus, βGK–Tag transgenic mice provide a new model system for studying the events that occur during both islet cell neogenesis and normal embryonic development.
Islets of Langerhans consist of aggregates of four distinct peptide hormone-secreting cell types [α, β, δ, and pancreatic peptide (PP)] dispersed throughout the pancreas. All four islet cell types, most of which are insulin-secreting β cells, originate from pluripotent progenitor cells both during embryonic development and later in life (1). Islet progenitor cells, which arise from the pancreatic ductal epithelium, undergo a series of cytodifferentiation steps that lead to the formation of mature islets (2). Although mature islet cells are well defined, the characteristics of islet progenitor cells, as well as the cell autonomous and epigenetic factors that control their cytodifferentiation, are not well understood, even though such knowledge may be necessary for the development of novel therapies for both type 1 and 2 diabetes mellitus.
During embryogenesis in rodents, presumptive islet precursor cells are first observed about halfway through the gestational period. These cells actively proliferate, differentiate, and then aggregate into mature islets, which are not seen until later in gestation (3). Postnatally, new islets continue to be formed but at a lower rate than during embryogenesis (3–5). In the adult, islet neogenesis is stimulated by certain chemical treatments, physical manipulations, and pathological conditions (6). Despite a lack of detailed information, there is evidence that the cytodifferentiation steps that occur during adult islet neogenesis represents a recapitulation of the same developmental events that occur during embryogenesis (3–5).
The recent cloning and functional characterization of transcription factors that bind to and transactivate genes encoding islet-specific peptides has provided useful markers for exploring pancreas and islet development. These include genes encoding the homeodomain protein Pdx-1, the basic helix–loop–helix factor Beta2/NeuroD, and the LIM/homeodomain protein Isl1. Mice that are homozygous null for these genes exhibit a range of defects in both pancreatic and islet morphogenesis as well as other developmental abnormalities. For instance, the absence of Pdx-1 results in pancreatic agenesis (7, 8), the absence of Beta2/NeuroD causes dysmorphic pancreatic islets and a diminished number and function of islet cells (9), and Isl1 knock-out mice completely lack differentiated islets (10). Potential roles for other key factors in islet development have been elucidated in a more fortuitous manner. For example, Pax4 and Pax6 homozygous null mice lack either β cell (11) or α cell (12) lineages, respectively, whereas Pax4/Pax6 double homozygous null mice have neither cell type (12). Other genes with expression highly restricted to the islet and/or β cell, such as the homeobox genes Nkx6.1 and Nkx2.2 (13), await similar genetic analysis, but they might also regulate terminal differentiation of β cells.
Previously, we observed that upstream glucokinase (βGK) promoter sequences direct reporter gene expression to a variety of rare neural/neuroendocrine (NE) cells, including both β cells and single, isolated ductal cells within the pancreas (14). By using βGK promoter sequences to direct the expression of simian virus 40 T antigen (Tag), an oncoprotein capable of transforming a variety of NE cells from discrete maturational stages (15), we have obtained a line of mice that develop NE cell tumors of the pancreas, pituitary, and stomach. Studies of the pancreatic tumors that arise in these animals revealed novel nonislet hormone-expressing tumors as well as typical insulinomas (16). Based on the characteristics of the hormone-negative tumors and their ability to cytodifferentiate in culture to islet hormone-producing cells, we suggest they are derived from islet progenitor cells.
METHODS
Construction of the GK–Tag Fusion Gene and Production of Transgenic Mice.
Simian virus 40 Tag sequences were removed from p1941B (provided by R. Hammer, Southwestern Medical Center, Dallas, TX) by digestion with BglII/BamHI and then ligated into pGK−1000/+14–luc after removal of luciferase sequences with BglII/BamHI. The βGK−1000/+14–Tag transgene was gel purified after digestion with KpnI/BamHI. Transgenic mice were generated by standard methods using (C57BL/6 × DBA/2)F2 embryos (17) and maintained by backcrossing to (C57BL/6 ×DBA/2)F1 mice.
Histologic and Immunohistochemical Analysis.
Tumors were removed, immersion fixed in 4.0% paraformaldehyde, and embedded in either paraffin or OCT compound as described previously (14). Both hematoxylin–eosin staining and immunohistochemistry were also performed as described previously (14). Peptide YY (PYY) antibody was obtained from Peninsula Laboratories. Antisera to Isl1 was obtained from S. Pfaff (Salk Institute). Antisera to mouse Pdx-1 was generated in rabbits by immunization with a purified glutathione S-transferase–N-terminal–Pdx-1 fusion protein. All secondary antibodies consisted of multiple-labeling grade donkey antispecies-specific IgG (Jackson Immunoresearch) conjugated to biotin, CY2, CY3, or CY5. Nuclear counterstaining with Yo–Pro1 (Molecular Probes) and confocal imaging was accomplished as described previously (8).
Whole-Mount in Situ Hybridization.
Digoxigenin-labeled sense and antisense RNA probes for localizing GK mRNA in early whole-mount embryos were synthesized using pGK.Z9, which contains a rat GK cDNA (18), as a template. Embryos were obtained from matings of ICR mice, dissected in PBS + 1% BSA and processed according to Hogan et al. (17). They were staged by considering noon of the day of the appearance of the vaginal plug as 0.5 days postcoitum. Following immunostaining and development in BM purple AP substrate (Boehringer Mannheim), the embryos were embedded in paraffin, sectioned, and counterstained with eosin. Images were recorded on Ektachrome, digitized with a Nikon slide scanner, and digitally negated and enhanced on a Silicon Graphics workstation running ImageWorks v.2.
Reverse Transcriptase (RT)–PCR Analysis.
RNA was prepared from pancreatic tumors using the RNeasy isolation kit (Qiagen, Chatsworth, CA). First-strand cDNA synthesis followed by multiplex RT–PCR was carried out essentially as described by Jensen et al. (13). The PCR primer pairs, PCR conditions, and cycle numbers used to assay gene expression are available upon request. Glucose-6-phosphate dehydrogenase or α-tubulin mRNAs were coamplified in each reaction as an internal control.
Cell Culture.
Pancreatic tumors were harvested from 150- to 180-day-old βGK–Tag mice, a small piece was removed and processed for immunohistochemistry, and cells were removed from the tumor interior and placed in single wells of 96-well tissue culture dishes with 100 μl of DMEM supplemented with 10% fetal calf serum, streptomycin, and penicillin (50 μg/ml each), diluted with an equal volume of filtered conditioned media obtained from a mouse insulinoma cell line. Cells were split at ≈70% confluency using 1% trypsin/EDTA.
RESULTS
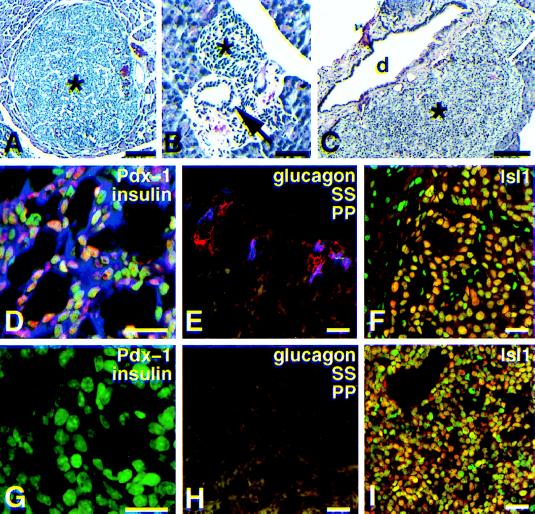
We produced 11 transgenic founder mice by using a βGK−1000/+14–simian virus 40 T antigen (Tag) fusion gene. Seven of these animals died within a few weeks of birth. Histochemical analysis of the tumors in the four founders that survived to breeding age revealed pancreatic, pituitary, and antral stomach tumors in all of the animals, with hepatobiliary and thyroid tumors also being rarely observed in one or more founders (Table 1). Only line 5, which had the most delayed tumor-related mortality (mean, 173 ± 27 days; n = 80), could be maintained past two generations. Studies of this sole surviving lineage revealed two major types of pancreatic tumors. Forty-nine percent (n = 73) were typical insulinomas (Fig. 1A) composed almost exclusively of insulin-immunopositive cells (Fig. 1D). The insulinomas appeared to arise from mature islets since non-β cells were often observed at the tumor periphery at a position characteristic of normal islet organization (Fig. 1E). Most of the remaining pancreatic tumors (51%) appeared to arise from hyperplastic cells in ductal regions (Fig. 1B) and lacked insulin immunoreactivity (Fig. 1G). Fifteen of 16 insulin-negative tumors did not show glucagon, pancreatic polypeptide, or somatostatin immunoreactivity (Fig. 1H), but one was a somatostatinoma. All of the pancreatic tumors lacked immunoreactivity for amylase, an acinar and ductal cell marker. Tag immunoreactivity was readily evident in nuclei of both tumors, but not in surrounding nonneoplastic cells. Both tumors had similar histologic appearances (Fig. 1 A and C) and both were immunopositive for the LIM/homeodomain factor Isl1 (10) (Fig. 1I). In addition, both tumor types expressed neuron-specific enolase, a general NE marker, and PYY, the earliest peptide hormone marker known for nascent islet cells (19), at similar frequencies by immunocytochemistry.
Table 1.
Location of tumors in βGK–Tag mice in the four founder animals that lived to a reproductive age
| Founder no. (age at death) | Tumor locations
|
||||
|---|---|---|---|---|---|
| Pancreas | Pituitary | Stomach | Hepatobiliary | Thyroid | |
| 5 | + | + | + | + | − |
| (173 days)* | |||||
| 12 | + | + | + | − | + |
| (90 days) | |||||
| 23 | + | + | + | − | + |
| (78 days) | |||||
| 78 | + | + | + | − | − |
| (141 days) | |||||
Average of 80 descendants.
Figure 1.
Histologic and immunochemical characterization of βGK−1000/+14–Tag pancreatic tumors. (A–C) Hematoxylin–eosin stained. (D–I) Confocal fluorescence imaging. (A) Overview of pancreas from βGK−1000/+14–Tag adult mouse showing a typical insulinoma (∗). (Bar, 100 μm.) (B) A periductal aggregate (∗) of hyperplastic epithelial cells, probably representing an early noninsulinoma tumor. A pancreatic duct is indicated by an arrow. (Bar, 50 μm.) (C) Noninsulinoma tumors (∗) were as prevalent as insulinomas, but more often observed near pancreatic ducts (d). (Bar, 0.2 μm). (D) Triple-stained insulinoma showing nuclear Pdx-1 immunoreactivity (orange nuclear color) in most insulin-positive cells (blue). Chromatin is stained green, which results in light green to bright orange color depending on the relative levels of Pdx-1. (Bar, 20 μm.) (E) Insulinoma stained for glucagon (blue), somatostatin (SS) (red), and pancreatic polypeptide (PP; green). Only a few cells located at the tumor periphery stain for these non-β cell markers. (Bar, 20 μm). (F) Insulinoma stained for Isl1 (orange nuclei). Nuclei are counterstained green. (Bar, 20 μm.) (G) Triple-stained undifferentiated tumor (compare with D) demonstrating lack of both PDX-1 and insulin immunoreactivity. Nuclei are counterstained green. (Bar, 20 μm.) (H) Lack of staining for peptide hormones in a noninsulinoma tumor (compare with E). (Bar, 20 μm.) (I) Noninsulinoma stained for Isl1 (orange; compare with insulinoma in F). Nuclei are counterstained green. Almost every cell in both the insulinomas and noninsulinomas stained for Isl1. (Bar, 20 μm.)
To explore the differences between the insulin-positive and -negative tumors, the expression of Pdx-1 was examined by immunocytochemical and immunoblot analysis. Interestingly, whereas all insulinoma tumors examined (n = 36) expressed this protein (Fig. 1D), Pdx-1 was only rarely observed in the noninsulinoma tumors (3 of 76 examined). The single somatostatinoma, noted above, also expressed Pdx-1.
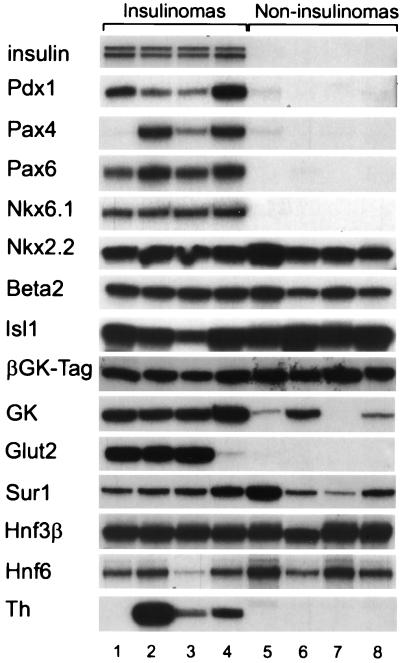
Both pancreatic tumor types were further analyzed using RT–PCR to determine whether genes known to be expressed in mature β cells were being expressed in the noninsulinomas (Fig. 2). Insulin mRNAs were absent in four noninsulinomas, but present in four insulinomas, as predicted by immunostaining. βGK–Tag mRNA was also present in both tumor types, confirming that tumorigenesis was associated with transgene expression. GK mRNA was also found in both tumor types, although at higher levels in insulinomas. mRNA encoding the sulfonylurea receptor, type 1 (Sur1) (20), a component of the ATP-sensitive K+ channel of β cells, was also present in both tumor types. In contrast, mRNAs for both Glut-2 and tyrosine hydroxylase, a marker for early islet progenitors (2, 21), was observed only in the insulinomas.
Figure 2.
RT–PCR characterization of gene expression in insulinomas and islet precursor cell (noninsulinoma) tumors from βGK–Tag mice. The RT–PCR amplification pattern of the indicated gene products are shown for eight different tumors, four of which were classified as insulinomas (lanes 1–4) and four as noninsulinomas (lanes 5–8) based on insulin immunostaining (Fig. 1). The insulinomas generally contained insulin (insulin II-top band, insulin I-bottom band), Pdx1, Pax4, Pax6, Nkx6.1, tyrosine hydroxylase (Th), and Glut-2 mRNAs, which were are not present in noninsulinoma tumors. Nkx2.2, Beta2/NeuroD, Isl1, simian virus 40 Tag, Sur1, GK, Hnf3β, and Hnf6 mRNAs were present in both tumor types.
We next analyzed both tumor types for expression of transcription factors with either established or postulated roles in pancreatic development (Fig. 2). mRNAs for both Pax6 (12) and Nkx6.1 (13) were expressed in all four insulinomas tested, whereas detectable levels of Pax4 mRNA (11) were present in three of the four insulinomas. These genes were either not expressed or expressed at markedly lower levels in the four noninsulinoma tumors tested. In contrast, the genes for Isl1 (10), Nkx2.2, Beta2/NeuroD (9), and the liver/endoderm-enriched transcription factors Hnf-3β, a winged helix transcription factor, and Hnf-6, a cut-like homeodomain factor, showed no consistent differences in expression between the two tumor types. Hnf-3β and Hnf-6 are both known to be expressed in β cells (22), and recent evidence suggests a potential hierarchical regulation of Pdx-1 by Hnf-3β in the pancreas (23) and of Hnf-3β by Hnf-6 in the liver (24).
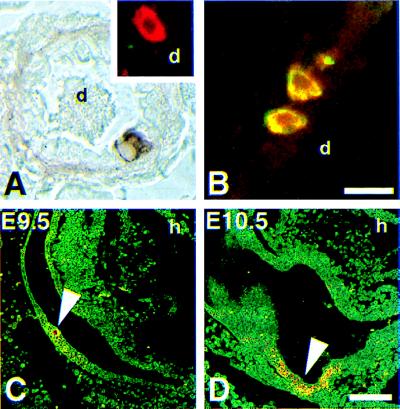
Comparing the similarities and differences in the gene expression profiles of the noninsulinomas to the insulinomas, we considered the possibility that the latter were derived from islet precursor cells. Pancreata from βGK−280/+14–hGH transgenic mice, which were previously characterized (14), were further analyzed and additional examples of βGK–hGH-expressing ductal cells were identified (Fig. 3A). Multiple-labeling immunofluorescence staining of several hGH-positive ductal cells revealed that these cells expressed PYY (Fig. 3B) but did not express either insulin or glucagon (Fig. 3A, Inset). Although there may be differences in the expression of Tag versus that of hGH due to the use of a slightly longer promoter fragment (−1000/+14 vs. −280/+14 bps), these results support the notion that Tag expression, and thus transformation, occurs within only a few ductal cells of the βGK–Tag mice.
Figure 3.
βGK−280/+14 transgene expression in pancreatic and presumptive islet rudiments in mice. (A and B) Immunofluorescence analysis of rare ductal cells that express βGK−280/+14–hGH transgene. (C and D) Whole-mount in situ hybridization of staged mouse embryos for GK mRNA. (A) βGK−280/+14–hGH is expressed in rare isolated cells and small clusters within the pancreatic duct (d) epithelium in adult mice. (Inset) Confocal image of a hGH-positive cell (red) proximal to a small ductule (d) that has been costained, but is negative for both insulin and glucagon. A pink, yellow, or whitish color would have indicated insulin and/or glucagon expression. (B) βGK−280/+14–hGH-expressing NE cells (red) along the duct (d) epithelia also stain for PYY (green) as revealed by their yellow emission. PYY immunoreactivity is restricted to α cells in adult islets (19). (Bar, 20 μm.) (C) Computer-enhanced reverse image of a mid-sagittal section through an E9.5 day embryo reveals a small cluster of hybridization signal (arrow) associated with thickened foregut epithelium in area destined to become dorsal pancreatic bud. Eosin counterstain. Heart (h) is indicated for reference. (D) Comparable section through an E10.5 day embryo showing hybridization signal (arrow) associated with dorsal pancreatic bud. GK mRNA was absent in liver rudiments in these early stages. (Bar, 100 μm.)
Given that islet neogenesis in the adult is thought to imitate the ontogenetic events that occur during embryogenesis, we next sought to determine whether βGK gene expression in the developing pancreas might be occurring at a time prior to that of the islet peptide hormone genes. By in situ hybridization Gk mRNA was detected among pancreatic rudiments beginning at E9.5 (Fig. 3 C and D). Hybridization was not observed at E8.5, consistent with time points reported by others for both mice and humans (25, 26), or in the liver rudiments, which is consistent with transcription originating from the upstream, NE-specific promoter in the GK gene.
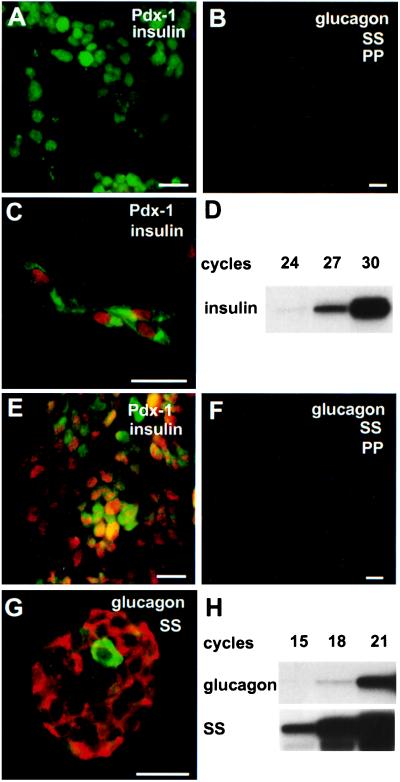
Although these results are consistent with the noninsulinomas arising from islet precursor cells, additional data in support of this possibility were obtained by attempting to propogate two different noninsulinoma tumors in cell culture. When removed from the animal, neither tumor expressed insulin, glucagon, somatostatin, or pancreatic polypeptide (Fig. 4 A, B, E, and F) by immunocytochemistry, although one tumor expressed Pdx-1 (Fig. 4E). When analyzed after several months in culture, the cells derived from these tumors were found to express one or more islet hormones. Tumor 5236C, which was Pdx-1 negative at the outset, remained almost totally Pdx-1 negative and hormone negative when examined at passages 10, 15, and 20 (≈7–24 wk in culture). However, at each passage a very small fraction of cells (<1%) developed both insulin and Pdx-1 coimmunoreactivity (Fig. 4C). These cells did not show evidence of somatostatin, glucagon, or PP gene expression after 30 cycles of RT–PCR. Insulin gene expression was detected beginning at 24 cycles (Fig. 4D), confirming the immunostaining. In contrast, cells from tumor 5230B, which was Pdx-1 positive upon necropsy and remained so in culture, were found to express either somatostatin or glucagon after 3 mo in culture. Using immunocytochemistry, somatostatin immunoreactivity was evident in nearly all of the cells derived from this tumor with glucagon also being present in a few rare cells when examined at passages 14, 18, and 19 (≈14–24 wk, Fig. 4G). The immunocytochemical staining result was confirmed by detection of somatostatin and glucagon mRNA at low RT–PCR cycle numbers (15 and 18 cycles, respectively, Fig. 4H). Pancreatic polypeptide mRNA was not detectable at 30 cycles in cells derived from either tumor (not shown), and insulin mRNA was not detectable at a high cycle number from cells grown from tumor 5230B. βGK–Tag mRNA remained readily detectable in cells from both tumors after culture.
Figure 4.
Cytodifferentiation of islet hormone-negative tumor cells in culture. (A–C and E–G) Confocal immunofluorescence analysis of tumors 5236C and 5230B and derived cells. (D and H) RT–PCR analysis of total RNA. (A) Lack of either insulin (blue) or Pdx-1 (red-orange) in section of tumor 5236C stained for both proteins. Neither insulin or Pdx-1 is expressed in this tumor. Nuclei are counterstained green. (B) Triple staining of tumor 5236C for glucagon (blue), somatostatin (SS, red), and pancreatic polypeptide (PP, green). Expression of these islet hormones was not detected, as was typical for almost all of the noninsulinomas. (C) Double staining for insulin (green) and Pdx-1 (red) of cells from this tumor after 16 wk in culture. Coexpression of insulin with Pdx-1 is seen in several cells. (D) RT–PCR confirming the expression of insulin I mRNA in total RNA isolated from cells derived from tumor 5236C. (E) Section of tumor 5230B, stained in the same way as A, showing expression of Pdx-1 but not insulin as judged by the red-orange nuclear signal and lack of a blue signal. (F) Triple staining of tumor 5230B, as in B, demonstrating the lack of glucagon, somatostatin, or PP immunoreactivity in the tumor at the time of necropsy. (G) Dual staining for glucagon (green) and somatostatin (red) of cells derived from tumor 5230B after 24 wk in culture. Almost all of the cultured tumor cells were found to express somatostatin, although rare glucagon-expressing cells were also observed. (H) RT–PCR showing presence of somatostatin and glucagon mRNAs in total RNA from cells derived from this tumor. (Bars, 20 μm.)
DISCUSSION
Both insulinomas and noninsulinomas were found to occur in equal frequency within the pancreas of a βGK–Tag transgenic mouse lineage that had delayed tumor onset (≈6 mo of age). The insulinomas appeared to be similar to those described for rat insulin promoter–Tag transgenic mice (16) and probably arose from terminally differentiated β cells. However, in contrast to rat insulin promoter–Tag transgenic mice, which only develop insulinomas, the βGK–Tag mice also developed tumors that, while in the animal, do not express any of the major islet peptide hormones. These tumors appear adjacent to pancreatic ducts, express only a subset of the genes that characterize mature β cells, and are capable of cytodifferentiation into hormone-producing cells when placed into culture.
Islet progenitor cells in the adult are a rare cell type whose characteristics are not defined. Islet cell progenitors are thought to reside within pancreatic ductal epithelium and to serve as a reservoir for islet neogenesis (4). Given such an origin for these cells, it is reasonable to expect that they express molecular markers shared between both the foregut endoderm and developing islet cells, that they express some of the transcription factors and other proteins that distinguish mature β cells, and that they are able to differentiate into islet hormone-expressing cells. By all of these criteria, the noninsulinoma tumors in the βGK–Tag mice appear to arise from islet progenitor cells.
The observation that some cells derived from the noninsulinoma tumors begin to make islet hormones when placed into culture provides the most compelling evidence that the tumors originated from islet progenitors. In two cases, tumors that lacked evidence of islet peptide hormone expression upon isolation from animals at least partially converted in culture to cells that expressed either insulin, somatostatin, or glucagon. These results are consistent with most current models of islet cell development that indicate the pluripotent nature of developing islet cells (2, 19, 21, 27, 28).
Islet cell differentiation depends on multiple transcription factors that display highly restricted cell-specific expression patterns (7–12). Gene inactivation experiments in mice suggest that each of these factors have overlapping, yet distinct, functions in the cytodifferentiation steps that lead to the formation of a mature islet. In this regard, it is significant that the transformed islet progenitor cells generally do not express several key transcription factors (Pdx1, Pax4, Pax6, and Nkx6.1) that currently seem to be essential for the terminal differentiation of β cells. Indeed, the incomplete gene expression repertoire of these islet progenitor cells provides novel insights into the complex transcriptional requirements and regulatory hierarchy required for the expression of certain genes in the mature β cell. For example, these results indicate that the expression of Sur1, GK, Nkx2.2, Beta2/NeuroD, Isl1, Hnf3β, and Hnf6 in the progenitor cells does not require, at least to any major extent, Pdx1, Pax4, Pax6, or Nkx6.1. In contrast, since Pdx-1 is known to transactivate both insulin (29, 30) and Glut-2 (31) gene expression, it is likely that the absence of Pdx1 in these ductal progenitor cells may underlie the lack of both of these genes in the precursor cells.
It is especially intriguing that the noninsulinomas lack Pdx-1 given that this homeodomain protein is widely expressed in the posterior foregut in early development (8). It is possible that the absence of Pdx-1 in islet progenitor cells may suspend these cells in an incompletely differentiated state. Later, expression of this protein may then allow the terminal steps of differentiation to proceed. The identification of a somatostatinoma that expressed Pdx-1 is also consistent with the reported role of this protein in transactivating the somatostatin gene (32, 33), although colocalization of Pdx-1 and somatostatin is fairly low in mature islets (34). Whether this Pdx-1/somatostatin-expressing tumor originated from a cell type at an intermediate stage in the cytodifferentiation pathway leading to the β cell, as suggested by one model of β cell neogenesis (35), remains to be further studied. Similarly, the observation that nearly all of the cells from tumor 5230B, which expressed Pdx-1 upon harvest, began to express either somatostatin or glucagon after several passages in culture also suggests an important role for this protein in determining the expression of both of these hormones.
The reason why two or more distinct pancreatic tumor types occur in βGK–Tag transgenic mice, compared with the formation of only insulinomas in rat insulin promoter–Tag mice, is unclear. However, the most likely explanation is that the βGK–Tag transgene is expressed at an earlier stage of islet development and/or neogenesis. Consistent with this is the detection of GK mRNA in the dorsal pancreatic rudiment at E9.5 at least as early as the first time reported for insulin expression (2, 36). The ability of the βGK promoter sequences to drive Tag expression to islet precursor cells suggests that other promoters may be similarly useful in generating tumors of islet precursor cells, perhaps even at different stages of cytodifferentiation. Such efforts might also be enhanced by using experimental strategies that limit the expression of Tag to specific lineages within the pancreas, thereby circumventing the morbidity problems associated with extrapancreatic tumors.
Although some heterogeneity in the gene expression profiles of various islet precursor cell tumors was observed, the βGK–Tag-induced tumors provide a more homogeneous source of these cells than has previously been achieved by other routes. For instance, the radiation-induced RIN (37) and MSL tumors (38, 39) have much more heterogeneous gene expression profiles. More importantly, the tumors that arise in the βGK–Tag mice appear to be less differentiated than the MSL tumors since all of the tumors, and the cells derived from them, express islet peptide hormones.
In conclusion, these studies provide strong evidence that we have transformed cells in the pancreas that are involved in islet neogenesis. Given that the rarity and dispersed nature of islet progenitor cells has proved a major hindrance to their characterization, we expect that the βGK–Tag mice and cell lines derived from them will facilitate the determination of the factors involved, target genes, temporal relationships, and transcriptional hierarchies that regulate islet cell cytodifferentiation.
Acknowledgments
We thank R. Stein and M. Gannon for reading the manuscript and for helpful comments, P. A. Labosky for experimental advice, and K. Newsom-Johnson for expert histologic assistance. These studies were supported by DK42612 and DK42502. J.M.M. was supported by a postdoctoral fellowship from the Juvenile Diabetes Foundation. Confocal images were acquired using the Cell Imaging Shared Resource (supported by Grants CA68485 and DK20593).
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
Abbreviations: GK, glucokinase; NE, neuroendocrine; PP, pancreatic peptide.
T.L.J. and J.M.M. contributed equally to this work.
References
- 1.LeDouarin N M. Cell. 1988;53:169–171. [Google Scholar]
- 2.Alpert S D, Hanahan D, Teitelman G. Cell. 1988;53:295–308. doi: 10.1016/0092-8674(88)90391-1. [DOI] [PubMed] [Google Scholar]
- 3.Gu D, Sarvetnick N. Development. 1993;118:33–46. doi: 10.1242/dev.118.1.33. [DOI] [PubMed] [Google Scholar]
- 4.Bonner-Weir S, Baxter L A, Schuppin G T, Smith F E. Diabetes. 1993;42:1715–1720. doi: 10.2337/diab.42.12.1715. [DOI] [PubMed] [Google Scholar]
- 5.Rafaeloff R, Pittenger G L, Barlow S W, Qin X F, Yan B, Rosenberg L, Duguid W P, Vinik A I. J Clin Invest. 1997;99:2100–2109. doi: 10.1172/JCI119383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Finegood D T, Scaglia L, Bonner-Weir S. Diabetes. 1996;44:249–256. doi: 10.2337/diab.44.3.249. [DOI] [PubMed] [Google Scholar]
- 7.Jonsson J, Carlsson L, Edlund T, Edlund H. Nature (London) 1994;371:606–609. doi: 10.1038/371606a0. [DOI] [PubMed] [Google Scholar]
- 8.Offield M F, Jetton T L, Labosky P A, Ray M, Stein R, Magnuson M A, Hogan B L M, Wright C V E. Development. 1996;122:983–995. doi: 10.1242/dev.122.3.983. [DOI] [PubMed] [Google Scholar]
- 9.Naya F J, Huang H, Qiu J, Mutoh H, DeMayo F J, Leiter A B, Tsai M. Genes Dev. 1997;11:2323–2334. doi: 10.1101/gad.11.18.2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ahlgren U, Pfaff S L, Jessell T M, Edlund T, Edlund H. Nature (London) 1997;385:257–260. doi: 10.1038/385257a0. [DOI] [PubMed] [Google Scholar]
- 11.Sosa-Pineda B, Chowdhury K, Torres M, Oliver G, Gruss P. Nature (London) 1997;386:399–402. doi: 10.1038/386399a0. [DOI] [PubMed] [Google Scholar]
- 12.St-Onge L, Sosa-Pineda B, Chowdhury K, Mansouri A, Gruss P. Nature (London) 1997;387:406–409. doi: 10.1038/387406a0. [DOI] [PubMed] [Google Scholar]
- 13.Jensen J, Serup P, Karlsen C, Nielsen T F, Madsen O D. J Biol Chem. 1996;271:18749–18758. doi: 10.1074/jbc.271.31.18749. [DOI] [PubMed] [Google Scholar]
- 14.Jetton T L, Liang Y, Pettepher C C, Zimmerman E C, Cox F G, Horvath K, Matchinsky F M, Magnuson M A. J Biol Chem. 1994;269:3641–3654. [PubMed] [Google Scholar]
- 15.Alarid A E, Windle J, Whyte D, Mellon P. Development Suppl. 1996;122:3319–3329. doi: 10.1242/dev.122.10.3319. [DOI] [PubMed] [Google Scholar]
- 16.Hanahan D. Nature (London) 1985;315:115–122. doi: 10.1038/315115a0. [DOI] [PubMed] [Google Scholar]
- 17.Hogan B L M, Beddington R, Constantini F, Lacy E. Manipulating the Mouse Embryo: A Laboratory Manual. 2nd Ed. Plainview, NY: Cold Spring Harbor Lab. Press; 1994. [Google Scholar]
- 18.Magnuson M A, Shelton K D. J Biol Chem. 1989;264:15936–15942. [PubMed] [Google Scholar]
- 19.Upchurch B H, Aponte G W, Leite A B. Development. 1994;120:245–252. doi: 10.1242/dev.120.2.245. [DOI] [PubMed] [Google Scholar]
- 20.Ashcroft F M, Harrison D E, Ashcroft S J H. Nature (London) 1984;312:445–448. doi: 10.1038/312446a0. [DOI] [PubMed] [Google Scholar]
- 21.Teitelman G, Lee J K. Dev Biol. 1987;121:454–466. doi: 10.1016/0012-1606(87)90182-5. [DOI] [PubMed] [Google Scholar]
- 22.Vaisse C, Kim J, Espinosa R, Beau M M L, Stoffel M. Diabetes. 1997;46:1364–1367. doi: 10.2337/diab.46.8.1364. [DOI] [PubMed] [Google Scholar]
- 23.Wu K-L, Gannon M, Peshavaria M, Offield M F, Henderson E, Ray M, Marks A, Gamer L W, Wright C V E, Stein R. Mol Cell Biol. 1997;17:6002–6013. doi: 10.1128/mcb.17.10.6002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Samadani U, Costa R H. Mol Cell Biol. 1996;16:6273–6284. doi: 10.1128/mcb.16.11.6273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bali D, Svetlanov A, Lee H-W, Fusco-DeMane D, Leiser M, Li B, Barzilai N, Surana M, Hou H, Fleischer N, Pinho R, Rosetti L, Efrat S. J Biol Chem. 1995;270:21464–21467. doi: 10.1074/jbc.270.37.21464. [DOI] [PubMed] [Google Scholar]
- 26.Mally M I, Otonkoski T, Lopez D, Hayek A. Pediatr Res. 1994;36:537–544. doi: 10.1203/00006450-199410000-00022. [DOI] [PubMed] [Google Scholar]
- 27.Teitelman G, Alpert S, Polak J M, Martinez A, Hanahan D. Development. 1993;118:1031–1039. doi: 10.1242/dev.118.4.1031. [DOI] [PubMed] [Google Scholar]
- 28.Herrera P-L, Huarte J, Zufferey R, Nichols A, Mermillod B, Philippe J, Muniesa P, Sanvito F, Orci L, Vassalli J-D. Proc Natl Acad Sci USA. 1994;91:12999–13003. doi: 10.1073/pnas.91.26.12999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ohlsson H, Karlsson K, Edlund T. EMBO J. 1993;12:4251–4259. doi: 10.1002/j.1460-2075.1993.tb06109.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Peers B, Leonard J, Sharma S, Teitelman G, Montminy M R. Mol Endocrinol. 1995;8:1798–1806. doi: 10.1210/mend.8.12.7708065. [DOI] [PubMed] [Google Scholar]
- 31.Waeber G, Thompson N, Nicod P, Bonny C. Mol Endocrinol. 1996;10:1327–1333. doi: 10.1210/mend.10.11.8923459. [DOI] [PubMed] [Google Scholar]
- 32.Leonard J, Peers B, Johnson T, Ferreri K, Lee S, Montminy M R. Endocrinology. 1993;7:1275–1283. doi: 10.1210/mend.7.10.7505393. [DOI] [PubMed] [Google Scholar]
- 33.Miller C P, McGehee R E, Habener J F. EMBO J. 1994;13:1145–1156. doi: 10.1002/j.1460-2075.1994.tb06363.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Guz Y, Montminy M R, Stein R, Leonard J, Gamer L W, Wright C V E, Teitelman G. Development. 1995;121:11–18. doi: 10.1242/dev.121.1.11. [DOI] [PubMed] [Google Scholar]
- 35.Teitelman G. Diabetes Metab Rev. 1996;12:91–102. doi: 10.1002/(SICI)1099-0895(199607)12:2<91::AID-DMR166>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 36.Gittes G K, Rutter W J. Proc Natl Acad Sci USA. 1992;89:1128–1132. doi: 10.1073/pnas.89.3.1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chick W L, Warren S, Chute R N, Like A A, Lauris V, Kitchen K C. Proc Natl Acad Sci USA. 1977;74:628–632. doi: 10.1073/pnas.74.2.628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Madsen O D, Larsson L-I, Rehfeld J F, Schwartz T, Lernmark A, Labrecque A, Steiner D F. J Cell Biol. 1986;103:2025–2034. doi: 10.1083/jcb.103.5.2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Madsen O D, Jensen J, Petersen H V, Andersen F G, Jensen P B, Jorgensen M C, Larsson L-I, Serup P. In: Pancreatic Growth and Regeneration. Sarvetnick N, editor. Basel, Switzerland: Karger Landes Systems; 1997. pp. 218–230. [Google Scholar]