Abstract
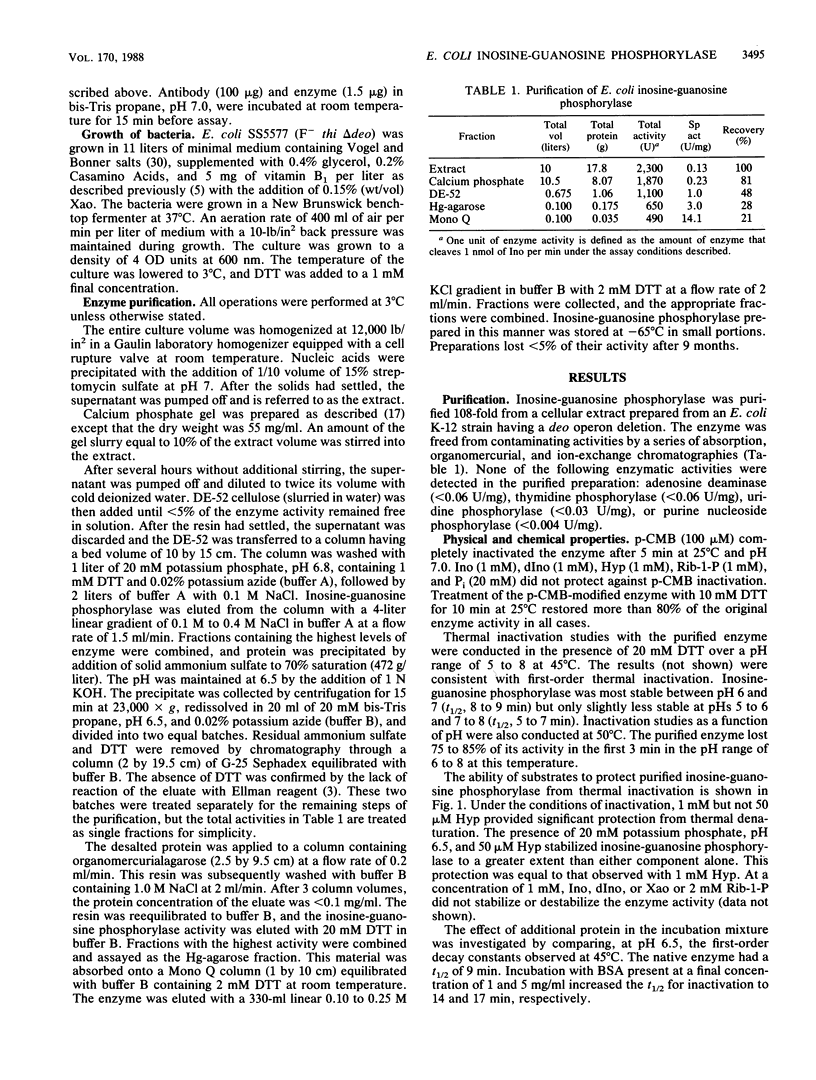
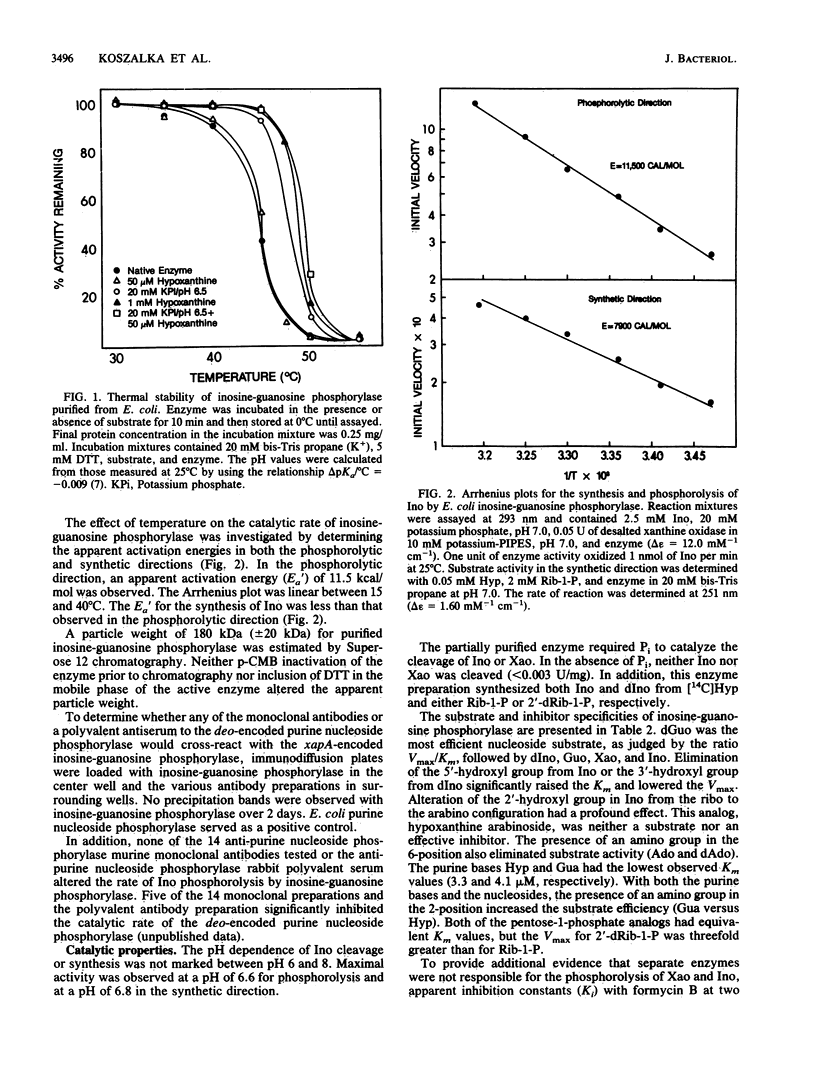
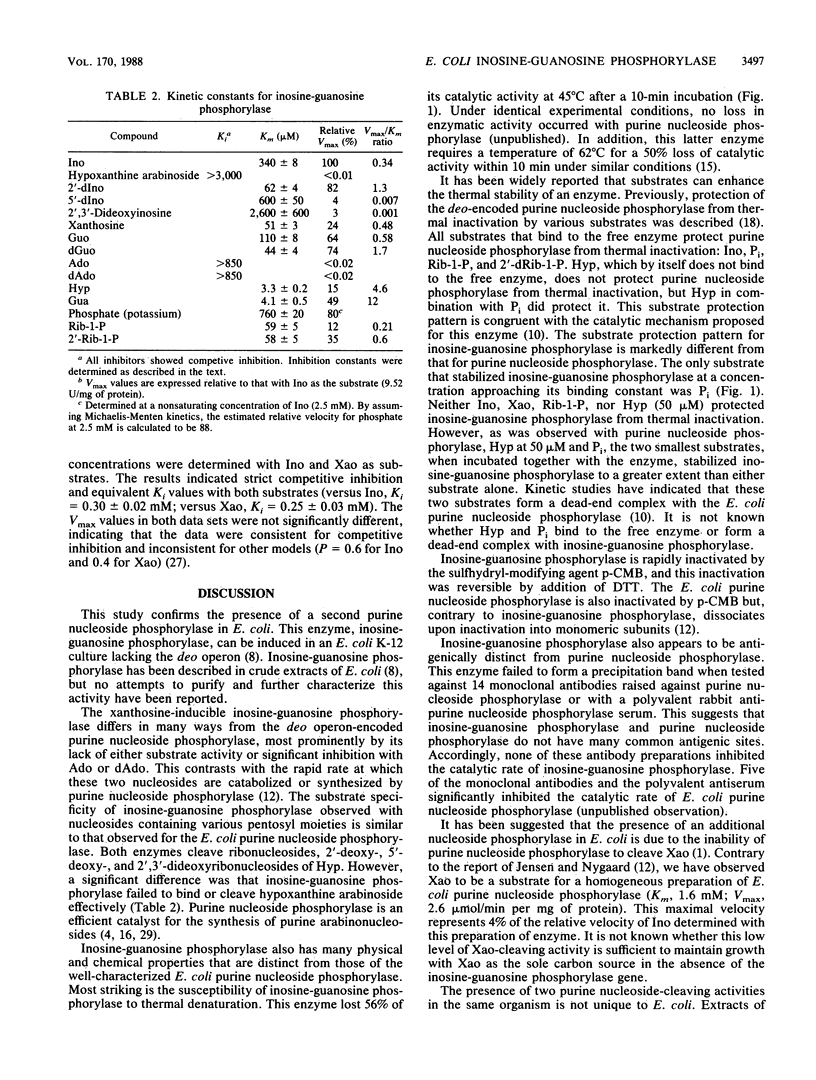
A xanthosine-inducible enzyme, inosine-guanosine phosphorylase, has been partially purified from a strain of Escherichia coli K-12 lacking the deo-encoded purine nucleoside phosphorylase. Inosine-guanosine phosphorylase had a particle weight of 180 kilodaltons and was rapidly inactivated by p-chloromercuriphenylsulfonic acid (p-CMB). The enzyme was not protected from inactivation by inosine (Ino), 2'-deoxyinosine (dIno), hypoxanthine (Hyp), Pi, or alpha-D-ribose-1-phosphate (Rib-1-P). Incubating the inactive enzyme with dithiothreitol restored the catalytic activity. Reaction with p-CMB did not affect the particle weight. Inosine-guanosine phosphorylase was more sensitive to thermal inactivation than purine nucleoside phosphorylase. The half-life determined at 45 degrees C between pH 5 and 8 was 5 to 9 min. Phosphate (20 mM) stabilized the enzyme to thermal inactivation, while Ino (1 mM), dIno (1 mM), xanthosine (Xao) (1 mM), Rib-1-P (2 mM), or Hyp (0.05 mM) had no effect. However, Hyp at 1 mM did stabilize the enzyme. In addition, the combination of Pi (20 mM) and Hyp (0.05 mM) stabilized this enzyme to a greater extent than did Pi alone. Apparent activation energies of 11.5 kcal/mol and 7.9 kcal/mol were determined in the phosphorolytic and synthetic direction, respectively. The pH dependence of Ino cleavage or synthesis did not vary between 6 and 8. The substrate specificity, listed in decreasing order of efficiency (V/Km), was: 2'-deoxyguanosine, dIno, guanosine, Xao, Ino, 5'-dIno, and 2',3'-dideoxyinosine. Inosine-guanosine phosphorylase differed from the deo operon-encoded purine nucleoside phosphorylase in that neither adenosine, 2'-deoxyadenosine, nor hypoxanthine arabinoside were substrates or potent inhibitors. Moreover, the E. coli inosine-guanosine phosphorylase was antigenically distinct from the purine nucleoside phosphorylase since it did not react with any of 14 monoclonal antisera or a polyvalent antiserum raised against deo-encoded purine nucleoside phosphorylase.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Buxton R. S., Hammer-Jespersen K., Valentin-Hansen P. A second purine nucleoside phosphorylase in Escherichia coli K-12. I. Xanthosine phosphorylase regulatory mutants isolated as secondary-site revertants of a deoD mutant. Mol Gen Genet. 1980;179(2):331–340. doi: 10.1007/BF00425461. [DOI] [PubMed] [Google Scholar]
- ELLMAN G. L. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959 May;82(1):70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- ERoshevskaia L. A., Barai V. N., Zinchenko A. I., Kvasiuk E. I., Mikhailopulo I. A. Preparativnyi sintez protivovirusnogo nukleozida 9-beta-D-arabinofuranoziladenina s pomoshch'iu bakterial'nykh kletok. Antibiot Med Biotekhnol. 1986 Mar;31(3):174–178. [PubMed] [Google Scholar]
- Fischer M., Short S. A. The cloning of the Escherichia coli K-12 deoxyribonucleoside operon. Gene. 1982 Mar;17(3):291–298. doi: 10.1016/0378-1119(82)90145-7. [DOI] [PubMed] [Google Scholar]
- Good N. E., Winget G. D., Winter W., Connolly T. N., Izawa S., Singh R. M. Hydrogen ion buffers for biological research. Biochemistry. 1966 Feb;5(2):467–477. doi: 10.1021/bi00866a011. [DOI] [PubMed] [Google Scholar]
- Hammer-Jespersen K., Buxton R. S., Hansen T. D. A second purine nucleoside phosphorylase in Escherichia coli K-12. II. Properties of xanthosine phosphorylase and its induction by xanthosine. Mol Gen Genet. 1980;179(2):341–348. doi: 10.1007/BF00425462. [DOI] [PubMed] [Google Scholar]
- Ives D. H., Durham J. P., Tucker V. S. Rapid determination of nucleoside kinase and nucleotidase activities with tritium-labeled substrates. Anal Biochem. 1969 Apr 4;28(1):192–205. doi: 10.1016/0003-2697(69)90170-5. [DOI] [PubMed] [Google Scholar]
- Jensen K. F., Nygaard P. Purine nucleoside phosphorylase from Escherichia coli and Salmonella typhimurium. Purification and some properties. Eur J Biochem. 1975 Feb 3;51(1):253–265. doi: 10.1111/j.1432-1033.1975.tb03925.x. [DOI] [PubMed] [Google Scholar]
- Jensen K. F. Purine-nucleoside phosphorylase from Salmonella typhimurium and Escherichia coli. Initial velocity kinetics, ligand banding, and reaction mechanism. Eur J Biochem. 1976 Jan 15;61(2):377–386. doi: 10.1111/j.1432-1033.1976.tb10031.x. [DOI] [PubMed] [Google Scholar]
- Jensen K. F. Two purine nucleoside phosphorylases in Bacillus subtilis. Purification and some properties of the adenosine-specific phosphorylase. Biochim Biophys Acta. 1978 Aug 7;525(2):346–356. doi: 10.1016/0005-2744(78)90229-2. [DOI] [PubMed] [Google Scholar]
- Koszalka G. W., Krenitsky T. A. Nucleosidases from Leishmania donovani. Pyrimidine ribonucleosidase, purine ribonucleosidase, and a novel purine 2'-deoxyribonucleosidase. J Biol Chem. 1979 Sep 10;254(17):8185–8193. [PubMed] [Google Scholar]
- Krenitsky T. A., Koszalka G. W., Tuttle J. V. Purine nucleoside synthesis, an efficient method employing nucleoside phosphorylases. Biochemistry. 1981 Jun 9;20(12):3615–3621. doi: 10.1021/bi00515a048. [DOI] [PubMed] [Google Scholar]
- Krenitsky T. A. Purine nucleoside phosphorylase: kinetics, mechanism, and specificity. Mol Pharmacol. 1967 Nov;3(6):526–536. [PubMed] [Google Scholar]
- Krenitsky T. A., Spector T., Hall W. W. Xanthine oxidase from human liver: purification and characterization. Arch Biochem Biophys. 1986 May 15;247(1):108–119. doi: 10.1016/0003-9861(86)90539-4. [DOI] [PubMed] [Google Scholar]
- Krenitsky T. A., Tuttle J. V. Correlation of substrate-stabilization patterns with proposed mechanisms for three nucleoside phosphorylases. Biochim Biophys Acta. 1982 May 3;703(2):247–249. doi: 10.1016/0167-4838(82)90055-3. [DOI] [PubMed] [Google Scholar]
- Krenitsky T. A., Tuttle J. V., Koszalka G. W., Chen I. S., Beacham L. M., 3rd, Rideout J. L., Elion G. B. Deoxycytidine kinase from calf thymus. Substrate and inhibitor specificity. J Biol Chem. 1976 Jul 10;251(13):4055–4061. [PubMed] [Google Scholar]
- Leer J. C., Hammer-Jespersen K., Schwartz M. Uridine phosphorylase from Escherichia coli. Physical and chemical characterization. Eur J Biochem. 1977 May 2;75(1):217–224. doi: 10.1111/j.1432-1033.1977.tb11520.x. [DOI] [PubMed] [Google Scholar]
- Miller R. L., Lindstead D. Purine and pyrimidine metabolizing activities in Trichomonas vaginalis extracts. Mol Biochem Parasitol. 1983 Jan;7(1):41–51. doi: 10.1016/0166-6851(83)90115-9. [DOI] [PubMed] [Google Scholar]
- Miller R. L., Sabourin C. L., Krenitsky T. A. Trypanosoma cruzi adenine nucleoside phosphorylase. Purification and substrate specificity. Biochem Pharmacol. 1987 Feb 15;36(4):553–560. doi: 10.1016/0006-2952(87)90366-2. [DOI] [PubMed] [Google Scholar]
- Schwartz M. Thymidine phosphorylase from Escherichia coli. Properties and kinetics. Eur J Biochem. 1971 Jul 29;21(2):191–198. doi: 10.1111/j.1432-1033.1971.tb01455.x. [DOI] [PubMed] [Google Scholar]
- Senesi S., Falcone G., Mura U., Sgarrella F., Ipata P. L. A specific adenosine phosphorylase, distinct from purine nucleoside phosphorylase. FEBS Lett. 1976 May 1;64(2):353–357. doi: 10.1016/0014-5793(76)80327-4. [DOI] [PubMed] [Google Scholar]
- Spector T., Hajian G. Statistical methods to distinguish competitive, noncompetitive, and uncompetitive enzyme inhibitors. Anal Biochem. 1981 Aug;115(2):403–409. doi: 10.1016/0003-2697(81)90025-7. [DOI] [PubMed] [Google Scholar]
- Spector T. Refinement of the coomassie blue method of protein quantitation. A simple and linear spectrophotometric assay for less than or equal to 0.5 to 50 microgram of protein. Anal Biochem. 1978 May;86(1):142–146. doi: 10.1016/0003-2697(78)90327-5. [DOI] [PubMed] [Google Scholar]
- Utagawa T., Morisawa H., Miyoshi T., Yoshinaga F., Yamazaki A., Mitsugi K. A novel and simple method for the preparation of adenine arabinoside by bacterial transglycosylation reaction. FEBS Lett. 1980 Jan 14;109(2):261–263. doi: 10.1016/0014-5793(80)81100-8. [DOI] [PubMed] [Google Scholar]
- VOGEL H. J., BONNER D. M. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956 Jan;218(1):97–106. [PubMed] [Google Scholar]
- WILKINSON G. N. Statistical estimations in enzyme kinetics. Biochem J. 1961 Aug;80:324–332. doi: 10.1042/bj0800324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman T. P., Gersten N. B., Ross A. F., Miech R. P. Adenine as substrate for purine nucleoside phosphorylase. Can J Biochem. 1971 Sep;49(9):1050–1054. doi: 10.1139/o71-153. [DOI] [PubMed] [Google Scholar]



