Abstract
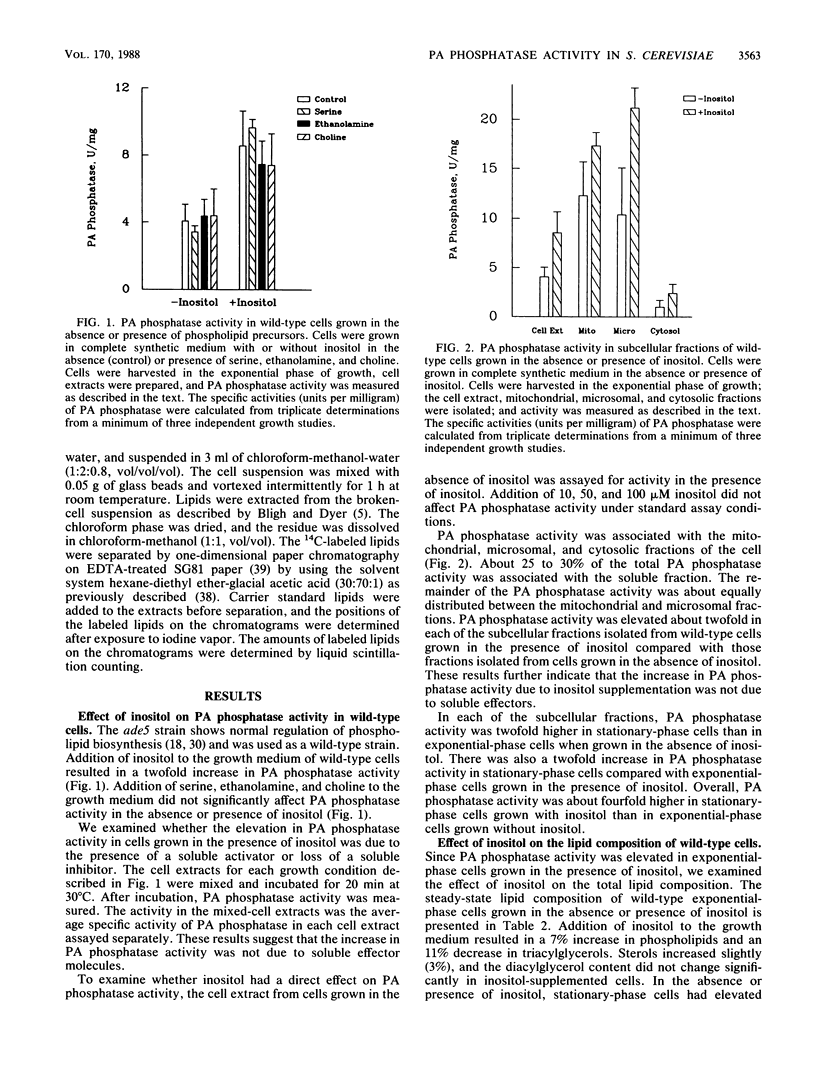
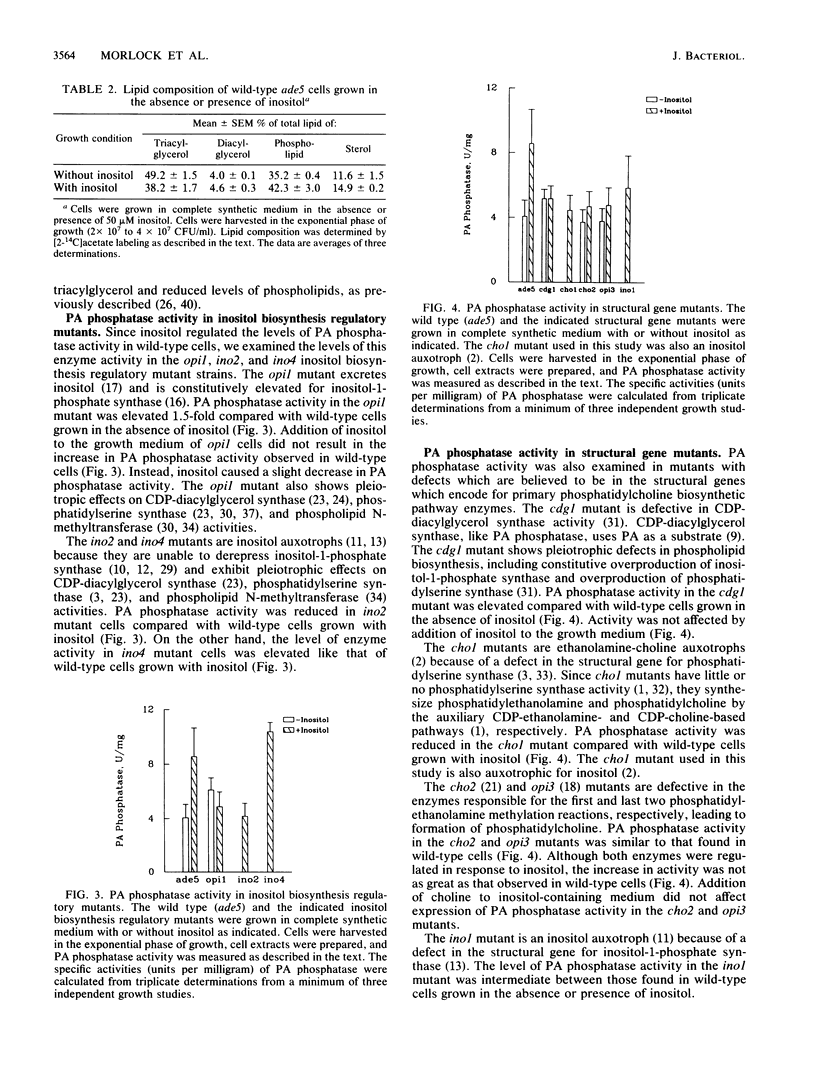
Regulation of phosphatidate phosphatase (EC 3.1.34) activity was examined in Saccharomyces cerevisiae cells supplemented with phospholipid precursors. Addition of inositol to the growth medium of wild-type cells resulted in a twofold increase in phosphatidate phosphatase activity. The increase in phosphatidate phosphatase activity was not due to soluble effector molecules, and inositol did not have a direct effect on enzyme activity. The phosphatidate phosphatase activity associated with the mitochondrial, microsomal, and cytosolic fractions of the cell was regulated by inositol in the same manner. Cells supplemented with inositol had elevated phospholipid levels and reduced triacylglycerol levels compared with unsupplemented cells. Serine, ethanolamine, and choline did not significantly affect the phosphatidate phosphatase activity of cells grown in the absence or presence of inositol. Enzyme activity was not regulated in inositol biosynthesis regulatory mutants, suggesting that regulation by inositol is coupled to regulation of inositol biosynthesis. Phosphatidate phosphatase activity was pleiotropically expressed in structural gene mutants defective in phospholipid biosynthesis. These results suggested that phosphatidate phosphatase was regulated by inositol at a genetic level.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atkinson K. D., Jensen B., Kolat A. I., Storm E. M., Henry S. A., Fogel S. Yeast mutants auxotrophic for choline or ethanolamine. J Bacteriol. 1980 Feb;141(2):558–564. doi: 10.1128/jb.141.2.558-564.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkinson K., Fogel S., Henry S. A. Yeast mutant defective in phosphatidylserine synthesis. J Biol Chem. 1980 Jul 25;255(14):6653–6661. [PubMed] [Google Scholar]
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Bailis A. M., Poole M. A., Carman G. M., Henry S. A. The membrane-associated enzyme phosphatidylserine synthase is regulated at the level of mRNA abundance. Mol Cell Biol. 1987 Jan;7(1):167–176. doi: 10.1128/mcb.7.1.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belendiuk G., Mangnall D., Tung B., Westley J., Getz G. S. CTP-phosphatidic acid cytidyltransferase from Saccharomyces cerevisiae. Partial purification, characterization, and kinetic behavior. J Biol Chem. 1978 Jul 10;253(13):4555–4565. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Carson M. A., Atkinson K. D., Waechter C. J. Properties of particulate and solubilized phosphatidylserine synthase activity from Saccharomyces cerevisiae. Inhibitory effect of choline in the growth medium. J Biol Chem. 1982 Jul 25;257(14):8115–8121. [PubMed] [Google Scholar]
- Carson M. A., Emala M., Hogsten P., Waechter C. J. Coordinate regulation of phosphatidylserine decarboxylase activity and phospholipid N-methylation in yeast. J Biol Chem. 1984 May 25;259(10):6267–6273. [PubMed] [Google Scholar]
- Carter J. R., Kennedy E. P. Enzymatic synthesis of cytidine diphosphate diglyceride. J Lipid Res. 1966 Sep;7(5):678–683. [PubMed] [Google Scholar]
- Culbertson M. R., Donahue T. F., Henry S. A. Control of inositol biosynthesis in Saccharomyces cerevisiae; inositol-phosphate synthetase mutants. J Bacteriol. 1976 Apr;126(1):243–250. doi: 10.1128/jb.126.1.243-250.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donahue T. F., Henry S. A. Inositol Mutants of SACCHAROMYCES CEREVISIAE: Mapping the ino1 Locus and Characterizing Alleles of the ino1, ino2 and ino4 Loci. Genetics. 1981 Jul;98(3):491–503. doi: 10.1093/genetics/98.3.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donahue T. F., Henry S. A. myo-Inositol-1-phosphate synthase. Characteristics of the enzyme and identification of its structural gene in yeast. J Biol Chem. 1981 Jul 10;256(13):7077–7085. [PubMed] [Google Scholar]
- Fischl A. S., Carman G. M. Phosphatidylinositol biosynthesis in Saccharomyces cerevisiae: purification and properties of microsome-associated phosphatidylinositol synthase. J Bacteriol. 1983 Apr;154(1):304–311. doi: 10.1128/jb.154.1.304-311.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl A. S., Homann M. J., Poole M. A., Carman G. M. Phosphatidylinositol synthase from Saccharomyces cerevisiae. Reconstitution, characterization, and regulation of activity. J Biol Chem. 1986 Mar 5;261(7):3178–3183. [PubMed] [Google Scholar]
- Greenberg M. L., Goldwasser P., Henry S. A. Characterization of a yeast regulatory mutant constitutive for synthesis of inositol-1-phosphate synthase. Mol Gen Genet. 1982;186(2):157–163. doi: 10.1007/BF00331845. [DOI] [PubMed] [Google Scholar]
- Greenberg M. L., Klig L. S., Letts V. A., Loewy B. S., Henry S. A. Yeast mutant defective in phosphatidylcholine synthesis. J Bacteriol. 1983 Feb;153(2):791–799. doi: 10.1128/jb.153.2.791-799.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg M. L., Reiner B., Henry S. A. Regulatory mutations of inositol biosynthesis in yeast: isolation of inositol-excreting mutants. Genetics. 1982 Jan;100(1):19–33. doi: 10.1093/genetics/100.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry S. A., Atkinson K. D., Kolat A. I., Culbertson M. R. Growth and metabolism of inositol-starved Saccharomyces cerevisiae. J Bacteriol. 1977 Apr;130(1):472–484. doi: 10.1128/jb.130.1.472-484.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry S. A., Klig L. S., Loewy B. S. The genetic regulation and coordination of biosynthetic pathways in yeast: amino acid and phospholipid synthesis. Annu Rev Genet. 1984;18:207–231. doi: 10.1146/annurev.ge.18.120184.001231. [DOI] [PubMed] [Google Scholar]
- Hirsch J. P., Henry S. A. Expression of the Saccharomyces cerevisiae inositol-1-phosphate synthase (INO1) gene is regulated by factors that affect phospholipid synthesis. Mol Cell Biol. 1986 Oct;6(10):3320–3328. doi: 10.1128/mcb.6.10.3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homann M. J., Bailis A. M., Henry S. A., Carman G. M. Coordinate regulation of phospholipid biosynthesis by serine in Saccharomyces cerevisiae. J Bacteriol. 1987 Jul;169(7):3276–3280. doi: 10.1128/jb.169.7.3276-3280.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homann M. J., Henry S. A., Carman G. M. Regulation of CDP-diacylglycerol synthase activity in Saccharomyces cerevisiae. J Bacteriol. 1985 Sep;163(3):1265–1266. doi: 10.1128/jb.163.3.1265-1266.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosaka K., Yamashita S. Partial purification and properties of phosphatidate phosphatase in Saccharomyces cerevisiae. Biochim Biophys Acta. 1984 Oct 24;796(1):102–109. [PubMed] [Google Scholar]
- Hosaka K., Yamashita S. Regulatory role of phosphatidate phosphatase in triacylglycerol synthesis of Saccharomyces cerevisiae. Biochim Biophys Acta. 1984 Oct 24;796(1):110–117. [PubMed] [Google Scholar]
- KATES M. Hydrolysis of lecithin by plant plastid enzymes. Can J Biochem Physiol. 1955 Jul;33(4):575–589. [PubMed] [Google Scholar]
- KENNEDY E. P., WEISS S. B. The function of cytidine coenzymes in the biosynthesis of phospholipides. J Biol Chem. 1956 Sep;222(1):193–214. [PubMed] [Google Scholar]
- Klig L. S., Henry S. A. Isolation of the yeast INO1 gene: located on an autonomously replicating plasmid, the gene is fully regulated. Proc Natl Acad Sci U S A. 1984 Jun;81(12):3816–3820. doi: 10.1073/pnas.81.12.3816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klig L. S., Homann M. J., Carman G. M., Henry S. A. Coordinate regulation of phospholipid biosynthesis in Saccharomyces cerevisiae: pleiotropically constitutive opi1 mutant. J Bacteriol. 1985 Jun;162(3):1135–1141. doi: 10.1128/jb.162.3.1135-1141.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klig L. S., Homann M. J., Kohlwein S. D., Kelley M. J., Henry S. A., Carman G. M. Saccharomyces cerevisiae mutant with a partial defect in the synthesis of CDP-diacylglycerol and altered regulation of phospholipid biosynthesis. J Bacteriol. 1988 Apr;170(4):1878–1886. doi: 10.1128/jb.170.4.1878-1886.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovác L., Gbelská I., Poliachová V., Subík J., Kovácová V. Membrane mutants: a yeast mutant with a lesion in phosphatidylserine biosynthesis. Eur J Biochem. 1980 Oct;111(2):491–501. doi: 10.1111/j.1432-1033.1980.tb04965.x. [DOI] [PubMed] [Google Scholar]
- Letts V. A., Klig L. S., Bae-Lee M., Carman G. M., Henry S. A. Isolation of the yeast structural gene for the membrane-associated enzyme phosphatidylserine synthase. Proc Natl Acad Sci U S A. 1983 Dec;80(23):7279–7283. doi: 10.1073/pnas.80.23.7279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loewy B. S., Henry S. A. The INO2 and INO4 loci of Saccharomyces cerevisiae are pleiotropic regulatory genes. Mol Cell Biol. 1984 Nov;4(11):2479–2485. doi: 10.1128/mcb.4.11.2479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCammon M. T., Parks L. W. Lipid synthesis in inositol-starved Saccharomyces cerevisiae. Biochim Biophys Acta. 1982 Oct 14;713(1):86–93. doi: 10.1016/0005-2760(82)90170-9. [DOI] [PubMed] [Google Scholar]
- Nikawa J. I., Yamashita S. Characterization of phosphatidylserine synthase from Saccharomyces cerevisiae and a mutant defective in the enzyme. Biochim Biophys Acta. 1981 Sep 24;665(3):420–426. doi: 10.1016/0005-2760(81)90254-x. [DOI] [PubMed] [Google Scholar]
- Poole M. A., Homann M. J., Bae-Lee M. S., Carman G. M. Regulation of phosphatidylserine synthase from Saccharomyces cerevisiae by phospholipid precursors. J Bacteriol. 1986 Nov;168(2):668–672. doi: 10.1128/jb.168.2.668-672.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotering H., Raetz C. R. Appearance of monoglyceride and triglyceride in the cell envelope of Escherichia coli mutants defective in diglyceride kinase. J Biol Chem. 1983 Jul 10;258(13):8068–8073. [PubMed] [Google Scholar]
- Steiner S., Lester R. L. Studies on the diversity of inositol-containing yeast phospholipids: incorporation of 2-deoxyglucose into lipid. J Bacteriol. 1972 Jan;109(1):81–88. doi: 10.1128/jb.109.1.81-88.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor F. R., Parks L. W. Triaglycerol metabolism in Saccharomyces cerevisiae. Relation to phospholipid synthesis. Biochim Biophys Acta. 1979 Nov 21;575(2):204–214. doi: 10.1016/0005-2760(79)90022-5. [DOI] [PubMed] [Google Scholar]
- Tillman T. S., Bell R. M. Mutants of Saccharomyces cerevisiae defective in sn-glycerol-3-phosphate acyltransferase. Simultaneous loss of dihydroxyacetone phosphate acyltransferase indicates a common gene. J Biol Chem. 1986 Jul 15;261(20):9144–9149. [PubMed] [Google Scholar]
- Waechter C. J., Lester R. L. Differential regulation of the N-methyl transferases responsible for phosphatidylcholine synthesis in Saccharomyces cerevisiae. Arch Biochem Biophys. 1973 Sep;158(1):401–410. doi: 10.1016/0003-9861(73)90637-1. [DOI] [PubMed] [Google Scholar]
- Waechter C. J., Lester R. L. Regulation of phosphatidylcholine biosynthesis in Saccharomyces cerevisiae. J Bacteriol. 1971 Mar;105(3):837–843. doi: 10.1128/jb.105.3.837-843.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh J. P., Bell R. M. sn-1,2-Diacylglycerol kinase of Escherichia coli. Mixed micellar analysis of the phospholipid cofactor requirement and divalent cation dependence. J Biol Chem. 1986 May 15;261(14):6239–6247. [PubMed] [Google Scholar]
- Yamashita S., Oshima A., Nikawa J., Hosaka K. Regulation of the phosphatidylethanolamine methylation pathway in Saccharomyces cerevisiae. Eur J Biochem. 1982 Nov 15;128(2-3):589–595. doi: 10.1111/j.1432-1033.1982.tb07005.x. [DOI] [PubMed] [Google Scholar]
- Yamashita S., Oshima A. Regulation of phosphatidylethanolamine methyltransferase level by myo-inositol in Saccaromyces cerevisiae. Eur J Biochem. 1980 Mar;104(2):611–616. doi: 10.1111/j.1432-1033.1980.tb04465.x. [DOI] [PubMed] [Google Scholar]



