Abstract
In social insects, colony-level complexity may emerge from simple individual-level behaviors and interactions. Emergent global properties such as colony size, which can be viewed as a consequence of life history traits, may influence individual-level behaviors themselves. The effects of colony size on productivity, body size, behavioral flexibility, and colony organization are examined here by considering colony size as an independent variable. Large colony size commonly corresponds with complex colony-level performance, small body size, and lower per capita productivity. Analyzing the construction behavior of various wasp societies reveals that complexity of individual behavior is inversely related to colony size. Parallel processing by specialists in large colonies provides flexible and efficient colony-level functioning. On the other hand, individual behavioral flexibility of jack-of-all trades workers ensures success of the small and early societies.
Evolutionary biology recently has developed new interest in explanations of how autonomous units can cooperate to form more complex systems (1). Parallel-processing systems poised at the boundary between chaos and order are well able to adapt and evolve (2). Parallel processing requires the existence of several agents or units, plus mechanisms that ensure the specialization and organization of these units into a complex efficient system. An intriguing and simple system organized in this way can be found in social insects, where the colony conducts all of its operations concurrently instead of sequentially. Reliability theory posits that redundancy at the subunit level is more efficient than redundancy at the system level (3). In relation to insect colonies, a system of redundant components (labor is divided by task, the separate tasks are concatenated to form a complete sequence) is preferred to several separate systems (wherein each individual performs the entire sequence independently of other individuals). Redundancy of workers leads to security because if one individual fails to complete a task, another may succeed, whereas a lone animal may lose all prior effort when one step in a sequence fails (4, 5). This holistic view subsumes explanations of three kinds that can be aligned with one another:
… the relative adaptiveness of the colonies as superorganismic operating units within their natural environment, the ergonomic matrix that determines an optimal or at least evolutionarily stable mix of castes and communication systems, and the details of the castes and communication systems themselves. (6)
In the current paper, we relate fundamental properties such as colony productivity, phylogenetic origins of small body size, and behavioral flexibility to colony size. We suggest that considerable changes in these properties are due to increase in colony size.
The Per Capita Paradox.
A fundamental paradox in insect societies is that, as colonies grow to contain more individuals, they generally appear to have lower per capita productivity (7). Why, then, if animals seem to produce offspring more efficiently alone or in small groups, do they form larger societies that may contain several million individuals (8)? Explanations for this paradox include: bias due to overlooking many small, failed colonies (7), kin selection (9, 10), females of low fitness joining groups, making these groups larger and lowering average fitness (11), protection from enemies (6, 12), and larger colonies have lower variance in productivity (13). Michener (7) also proposed that, to achieve small increase in the number of reproductives, a colony must invest much more in terms of workers. This phenomenon may drive the system toward large colony size especially when workers are long lived. Michener (7) found two exceptions to the general pattern described above, Bombus and Pseudagapostemon. In the first case, he explained the discrepancy by the necessity of having many workers per larva to rear reproductive brood (thus, depressing the productivity of small colonies); in the second case, where there is no caste among females nor cooperative activity, constant reproductivity per female occurs (a situation we regard as confirming rather than challenging his general argument because it represents the uniformity of individual effort).
Recently, Jeanne and Nordheim (14) suggested that Michener’s (7) results on swarm founding wasps may be an artifact of his need to lump colonies of different species and development stages to increase sample size. They proposed that per capita output actually increases with swarm size in Polybia occidentalis. Such a divergence from the general patterns Michener established needs to be examined closely. The colonies of Jeanne and Nordheim (14) were absconding swarms that survived experimental destruction of the nest, and they were observed only at the 25th day after initiating a new nest (before new adults emerge). Seventeen of their 21 data points represent nests in the lowest 1/4 of the range of colony size of this species. Of the four larger nests, two of low productivity were eliminated as outliers, thus the two remaining colonies serve to predict the relationship for 3/4 of the range of colony size. Instead of calculating per capita variables, the authors used a curve fitting technique including second and third order polynomials that are not biologically interpretable. Results from earlier work (15) were used to explain a mechanism that would account for this pattern: shorter time spent waiting for pulp to be downloaded from a forager to a builder as colony size increases. Although efficiency in such transfers is important in construction behavior (below), gaining some seconds in queuing time during construction is not translated easily into differences in fitness-related variables like brood weight, mainly because construction behavior precedes egg laying and lasts only a few days (16).
We calculated per capita values from the data of Jeanne and Nordheim (14) and found that per capita output does not increase with colony size (contra 14). Per capita output instead shows large variance, especially in small colony sizes (Fig. 1). These results agree with Michener’s rule (7) and can be explained by application of central limit theorem (13). Small samples (few foragers collecting for small colonies) are more likely to show large deviations from the expected mean and are less predictable than large samples (many workers collecting for large colonies) (17). Wenzel and Pickering (13) explored this by using a statistical model and data for foraging. They supposed that production schedules are based on the variance rather than the mean. Higher per capita numbers of brood in small colonies may be a selected response to unpredictability of resources. The costs of this are shown by abortion of eggs and larvae before maturity or longer larval development time (13). Under good conditions, larger groups have lower per capita productivity, but in bad conditions they lose less brood by abortion and may suffer less jeopardy of catastrophic nest loss or adult mortality (18). A larger number of workers decreases variance in part through more frequent scanning of the environment and higher interaction rates among workers.
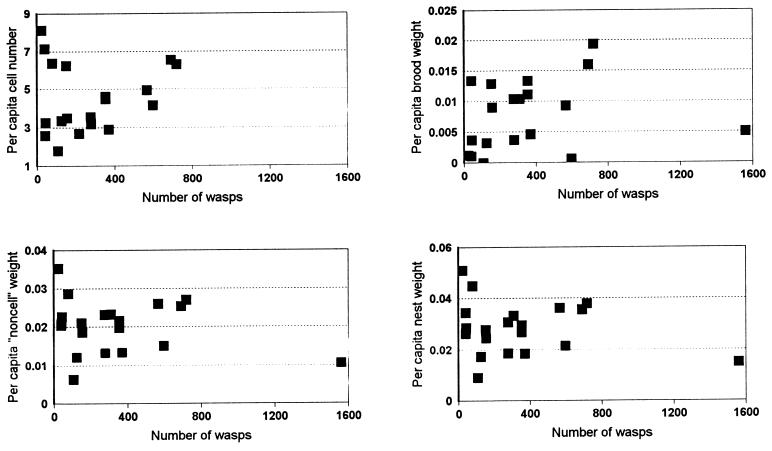
Figure 1.
Per capita productivity of Polybia occidentalis. Per capita values calculated from raw data of table 1 in ref. 14. Although the data are inappropriately distributed for regression analysis (“funnel” shaped distribution), it is clear that there is not an increase in productivity with colony size, contra Jeanne and Nordheim’s (14) conclusion based on curve-fitting.
Individual vs. Colony Level Flexibility.
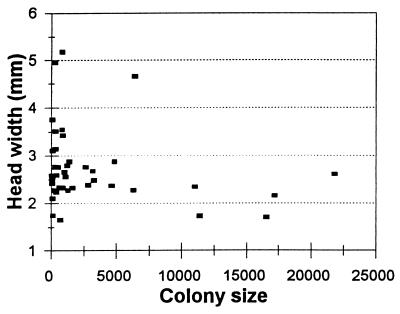
By using optimization models, Oster and Wilson (19) supposed that a positive correlation exists between behavioral tempo and the mature colony size of ant species. Maintaining high tempo requires either changing local density within a small colony (20) or increasing colony size. One way to increase colony size (and therefore tempo of interaction) is by reducing body size. With no fundamental change in ecological constraints or energy budgets, a colony can produce more, smaller individuals in place of fewer, larger ones, increasing the tempo of interaction simply by increasing the number of individuals present. Plotting head width of workers against largest known colony size for different species of swarming wasps (Fig. 2) illustrates that the species with largest colony size have relatively small body size (particularly some Polybia, Protopolybia, and swarming species of Ropalidia, commonly less than 2 mm head width), and species of large body size nearly always have smaller colonies. Unfortunately, standard statistical examination of variables in Fig. 2 (such as regression analysis) is not possible because species are related, and differentially so. Evolutionary histories are partly shared depending on lineage, so the fundamental assumption of independence among samples is violated (24, 25). In addition to the data shown in Fig. 2, the largest social wasps (with head width of 4 mm or more) are commonly found in the primitively social genera Polistes, Mischocyttarus, and Belonogaster in which single females initiate colonies that generally do not grow above 50 (or rarely 100) contemporaneous workers. Thus, it appears that small body size is associated with swarming habit.
Figure 2.
Head width and largest known mature colony size for swarming Polistinae. Colony size taken from table 6.5 in ref. 21, except for Protonectarina sylveirae (22) and Polybioides raphigastra (23). Representative head widths measured with a dissection microscope ocular micrometer across the widest part of the eyes for a single characteristic worker, to the nearest 0.01 mm, from museum specimens: Agelaia areata 2.61; A. cajennensis 2.42; A. fulvofasciata 2.88; A. lobipleura 2.59; Angiopolybia pallens 2.23; Apoica pallens 3.14; Brachygastra augusti 2.38; B. scutellaris 2.33; Chartergellus atectus 2.49; Chartergellus communis 2.77; Chartergus chartarius 2.68; Leipomeles dorsata 1.74; Metapolybia azteca 2.56; M. cingulata 2.53; Clypearia sulcata 3.11; Parachartergus fraternus 2.76; Polybia bicyttarella 2.11; P. bistriata 2.11; P. catillifex 2.58; P. dimidiata 4.66; P. emaciata 2.58; P. erythrothorax 2.27; P. jurinei 2.66; P. occidentalis 2.32; P. parvulina 2.34; P. platycephala 2.34; P. rejecta 2.87; P. ruficeps 2.37; P. scutellaris 2.27; P. sericea 3.44; P. singularis 2.80; P. striata 3.55; Polybioides raphigastra 2.76; Protopolybia acutiscutis 1.70; P. minutissima 1.65; P. sedula 1.73; Pseudopolybia compressa 3.52; P. vespiceps 3.77; Ropalidia kurandae 2.27; R. romandi 2.15; R. trichophthalma 2.49; Synoeca septentrionalis 4.97; S. surinama 5.18; Protonectarina sylveirae 2.35.
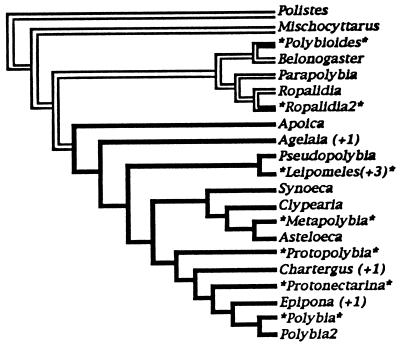
One way to test for a relationship between body size and swarming habit is to consider them as binary variables in Maddison’s (26) concentrated changes test. To account for phylogenetic effects directly, this procedure examines the probability of finding the observed association between an independent variable (swarming) and a dependent variable (reduced body size), given an explicit phylogeny and the assumption that evolutionary change in the dependent variable is equiprobable across all nodes of the phylogeny. Application of the test (Fig. 3) finds marginal significance for the distribution of larger or smaller wasps, whether optimizing as separate derivations of small size (parsimonious, and preferred) or as fewer derivations and several reversals (more evolutionary events, and therefore more ad hoc). Thus, evolution of swarming habit was followed by repeated and independent reduction in body size. We interpret this as evidence of the importance of maintaining high tempo of interaction.
Figure 3.
Maddison’s (26) concentrated changes test examining the correlation between reduced body size (starred genera) and swarming habit (black lineages), 10,000 replicate simulations by using macclade ver. 3. Phylogeny from ref. 25, dividing out swarming Ropalidia (Ropalidia2) and small Polybia (Polybia2), marrying together monophyletic combinations of genera that are similar in size (Agelaia combined with Angiopolybia; Leipomeles combined with Chartergellus, Marimbonda, and Nectarinella; Chartergus combined with Brachygastra; Epipona with Synoecoides). This topology requires (most parsimoniously and optimal) seven separate reductions of body size in the starred terminals (P = 0.051) or (less parsimoniously, and less preferred) six reductions followed by two reversals to larger size (P = 0.056) or five reductions and three reversals (P = 0.058).
It is reasonable to assume that tempo and caste complexity should be related, even if only indirectly through colony size, as Wilson (8) has proposed for queen/worker dimorphism. Frequent interaction also permits (or is a necessary precursor for) the evolution of more complex castes and division of labor (27). Oster and Wilson (19) also proposed that the probability that a caste can perform a task with adequate competence increases abruptly when the behavioral flexibility of the caste reaches a certain level. This phenomenon is reminiscent of “phase transition” curves, which characterize sudden condensation, shift from order to disorder, and other abrupt changes of important magnitude. For example, in ants, the shift from polymorphism to monomorphism can occur with a relatively small increment in either behavioral flexibility or the capacity to work cooperatively within the same caste. The generality of these models outside of ants has not been tested previously, but here we demonstrate its legitimacy in social wasps that lack multiple morphological castes of workers. Focusing on nest building, we show that every element of complex construction behavior and organization of work found in large colonies also exists in the most primitive and smallest societies, but individual flexibility differs.
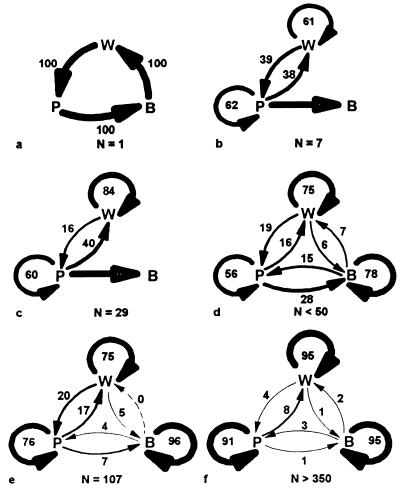
Construction behavior requires two materials collected from the environment (water and cellulose pulp) and two classes of behaviors (foraging and building). When a single wasp performs the duty, the building sequence is predetermined. First, the wasp collects water, then it flies to the pulp source where it collects pulp, then it returns to the nest, and finally it builds the pulp into the nest structure (Fig. 4a). When more wasps are present (e.g., in a postemergence nest), inter-individual differences in behavior frequencies generally emerge. One component is age polyethism, in which younger individuals usually remain on the nest and older ones carry out foraging (refs. 31 and 32; the queen is exceptional in this regard because she rarely leaves the nest after workers emerge). Also, with the exception of Vespinae (33, 34), as colony size increases, part of the pulp arriving at the nest is shared between nestmates (Table 1). Thus, wasps that did not collect pulp take part in building behavior (37). This is especially true for the queen, who solicits water and pulp from other wasps on the nest and then uses these materials mainly for cell initiation (29, 36, 35). Specialization emerges among foragers too, where foraging for food or water is highly specialized (individuals rarely switch to another material), in contrast to pulp foragers that switch to another material almost every other trip (28, 29).
Figure 4.
Frequency of transitions between three construction tasks, building (B), pulp foraging (P), and water foraging (W). (a) Single foundress, as in Polistes. (b) Vespula sylvestris, colony of seven individuals, 44 transitions of one individual, from ref. 28. (c) Polistes fuscatus, colony of 29 individuals, 155 transitions, recalculated from ref. 29. (d) Polybia occidentalis, data pooled from four colonies of <50 individuals, 797 transitions (15). (e) Metapolybia mesoamerica (30), colony of 107 individuals, 117 transitions (this study). (f) Polybia occidentalis, data pooled from three colonies larger than 350 individuals, 2,085 transitions, from ref. 15. Width of arrows corresponds to frequency; numerals indicate exact values. In b and c, every pulp forager also built with her pulp (regardless of sharing) as indicated by the large, straight arrow.
Table 1.
Pulp handling in Polistinae wasps
| Species | Colony size | Keep | Divide | Give | Sample size | Ref. |
|---|---|---|---|---|---|---|
| Polistes fuscatus | 1–7 | 0.93 | 0.07 | 0 | 305 | 35 |
| Polistes instabilis | 1–8 | 0.74 | 0.26 | 0 | 100 | 35 |
| Miscocyttarus drewseni | 5–27 | 0.80 | 0.20 | 0 | 195 | 36 |
| Polistes fuscatus | 10–20 | 0.49 | 0.45 | 0.06 | 203 | 37 |
| Polybia occidentalis | <50 | 0 | 0.18 | 0.82 | 485 | 15 |
| Metapolybia aztecoides | 63 | 0 | 0.06 | 0.94 | 35 | This study |
| Metapolybia mesoamerica | 107 | 0 | 0.02 | 0.98 | 55 | This study |
| Polybia occidentalis | >350 | 0 | 0.002 | 0.992 | 920 | 15 |
Pulp sharing increases with colony size, either by dividing the pulp between the forager and a builder or by giving the entire load to a builder. In larger colonies, the pulp forager gives away the full load more frequently as colony size increases, with this load subsequently divided among several builders.
The only qualitative difference that can be found between the building behavior of independent- versus swarm-founding wasps is that pulp foragers of swarm-founding wasps never keep the whole load of pulp to build with it (Table 1). The pulp load is generally so large that it cannot be processed by a single individual. If the pulp is not accepted by any wasps, the whole load is discarded (I.K., unpublished personal observation on Metapolybia). We propose that increased load size is a secondary consequence of the emergence of a more complex building behavior.
Normally, the pulp forager shares the load with nestmates or gives the whole load to another individual that will share the load with others (Table 1). When a pulp forager gives the whole load to another wasp, it does not take part in the building behavior per se; it only forages for the material. As colony size increases, it is less probable that the pulp forager will take part in building. Similar trends can be seen if we study the transitions between all behaviors connected to construction behavior (Fig. 4). In small colonies, there are frequent transitions between pulp foraging and water foraging, as well as between pulp foraging and building. As colony size increases, specialization becomes more evident. Builders seem to be the most specialized group in every case. This might be the consequence of age polyethism (32), i.e., they are younger than foragers and tend to remain on the nest, whereas foragers may revert to nest-bound behavior if colony needs require it. In addition, O’Donnell (39) proposed that larger colonies can support genetically differentiated specialists.
The cues that govern the organization of construction behavior are only partly known. Jeanne (16) concluded that construction is controlled by feedback across task groups (not from the nest or taskmates). The pulp–forager group adjusts its rate of activity to the demand for pulp as set by the builders (who have regular contact with the structure of the nest), and water foragers are less responsive because “water foragers are two steps removed from the ultimate source of information” (16). We found no evidence for this information flow, either in Metapolybia (unpublished work) or in primitive societies such as Polistes (5, 40). Organization of work is instead related to one (or more) variables corresponding to colony size. One such variable would be the rate of interaction between colony members, which, as it rises with colony size, allows more frequent scanning of the environment and of colony needs. High rates of interaction also permit the development of parallel processing systems. The question is how this parallel processing system emerges, and how is it controlled for adaptive performance? In a separate, unpublished paper, we propose that construction is constrained by the capacity of colony to store water. If collecting water from nestmates is difficult, neither pulp forager nor builder can specialize because water is needed for both tasks. These wasps have to collect water or they have to wait until there is enough water transported by others for these duties. As colony size increases, the colony is able to store more water in the crops of the larger number of individuals, which serves to buffer fluctuation in supply. When the colony exceeds several hundred individuals, parallel processing by highly specialized units emerges. We do not claim that colony size alone determines the difference between the building behaviors in certain species; we simply suggest that differences do not necessarily result from gross differences at the behavioral level (41) but rather from a variable (or several variables) corresponding to colony size.
Strategies for Efficient Colony Level Performance.
From these findings, it seems that social insects can cope with variable environments through different behavioral solutions that correspond to colony size. Whereas the intuitive relationship might be that behavioral specialization leads to the success that produces large colony size, we argue that the reverse is likely in certain contexts: Large colony size is a prerequisite for behavioral specialization (which subsequently may afford increased success). At one end of the spectrum, individually founded, small, short-lived colonies are most likely to have risk-tolerant caste proportions and behaviors, which is to say less canalized caste composition. They are adapted to absorbing large fluctuations in the environment, either in the material supply or in attack by predators, by relying on more generalists in the work force. Oster and Wilson (19) characterized such workers as being large, slow, and more “careful,” that is, acting with greater deliberateness and precision. At the other end of the spectrum are species that capitalize on rapid growth of a large work force. These species sacrifice per capita productivity to decrease variance in colony performance. Such a strategy relies on a high rate of exploration and exploitation of the environment by numerous small, fast workers. Large colony size allows redundant, parallel organization yielding higher system-level reliability that rests on specialized workers performing compartmentalized tasks. If several workers in a caste fail in their duties, success by others in the same caste make up for the shortfall. Where a species falls in the continuum of individual versus colony-level flexibility is tuned evolutionarily through life history parameters corresponding to colony size.
Acknowledgments
We thank two anonymous reviewers for helpful comments on the early version of the manuscript, M. E. Smethurst and J. M. Carpenter for describing Metapolybia mesoamerica, and J. M. Carpenter for providing some head width measurements. This work was suppported by the Szechenyi István Scholarship Fund, the Hungarian National Scientific Foundation (OTKA F-020572) (I.K.), the U.S.–Hungarian Science and Technology Joint Fund (JF no. 350) (I.K., J.W.W.), and the Ohio State University Research Foundation (J.W.W.).
References
- 1.Maynard Smith J, Szathmáry E. The Major Transitions of Evolution. Spektrum, Oxford: Freeman; 1995. [Google Scholar]
- 2.Kaufman S A. The Origins of Order: Self-Organization and Selection in Evolution. New York: Oxford Univ. Press; 1993. [Google Scholar]
- 3.Barlow R, Proschan F. Statistical Theory of Reliability and Life Testing. Rinehart, and Winston, New York: Holt; 1975. [Google Scholar]
- 4.Smith A P. Anim Behav. 1978;26:232–240. [Google Scholar]
- 5.Karsai I, Theraulaz G. Sociobiology. 1995;26:83–114. [Google Scholar]
- 6.Hölldobler B, Wilson E O. The Ants. Cambridge, MA: Belknap; 1990. [Google Scholar]
- 7.Michener C D. Insectes Sociaux. 1964;11:317–341. [Google Scholar]
- 8.Wilson E O. The Insect Societies. Cambridge, MA: Belknap; 1971. [Google Scholar]
- 9.Hamilton W D. J Theoret Biol. 1964;7:1–16. doi: 10.1016/0022-5193(64)90038-4. [DOI] [PubMed] [Google Scholar]
- 10.Hamilton W D. J Theoret Biol. 1964;7:17–52. doi: 10.1016/0022-5193(64)90039-6. [DOI] [PubMed] [Google Scholar]
- 11.West Eberhard M J. J Kansas Entomol Soc. 1978;51:832–856. [Google Scholar]
- 12.Michener C D, Brothers D J. Proc Natl Acad Sci USA. 1974;71:671–674. doi: 10.1073/pnas.71.3.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wenzel J W, Pickering J. Proc Natl Acad Sci USA. 1991;88:36–38. doi: 10.1073/pnas.88.1.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jeanne R L, Nordheim E V. Behav Ecol. 1996;7:43–48. [Google Scholar]
- 15.Jeanne R L. Behav Ecol Sociobiol. 1986;19:333–341. [Google Scholar]
- 16.Jeanne R L. Anim Behav. 1996;52:473–488. [Google Scholar]
- 17.Sokal R R, Rohlf F J. Biometry. San Francisco: Freeman; 1974. [Google Scholar]
- 18.Queller D C. Proc Natl Acad Sci USA. 1989;86:3224–3226. doi: 10.1073/pnas.86.9.3224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oster G F, Wilson E O. Caste and Ecology in the Social Insects. Princeton: Princeton Univ. Press; 1978. [PubMed] [Google Scholar]
- 20.Gordon D M, Paul R E H, Thorpe K. Anim Behav. 1993;45:83–100. [Google Scholar]
- 21.Jeanne R L. In: The Social Biology of Wasps. Ross K G, Matthews R W, editors. Ithaca, NY: Cornell Univ. Press; 1991. pp. 191–231. [Google Scholar]
- 22.Shima S N, Yamane S, Zucchi R. Bull Fac Educ Ibaraki Univ (Nat Sci) 1996;45:57–67. [Google Scholar]
- 23.Vecht J v d. Zool Verhand (Leiden) 1966;82:1–42. [Google Scholar]
- 24.Felsenstein J. Am Nat. 1985;125:1–15. [Google Scholar]
- 25.Wenzel J W, Carpenter J M. In: Phylogenetics and Ecology. Eggleton P, Vane-Wright R I, editors. London: Academic; 1994. pp. 79–101. [Google Scholar]
- 26.Maddison W P. Evolution. 1990;44:539–557. doi: 10.1111/j.1558-5646.1990.tb05937.x. [DOI] [PubMed] [Google Scholar]
- 27.Bonabeau E, Theraulaz G, Deneubourg J L, Aron S, Camazine S. Trends Ecol Evol. 1997;12:188–193. doi: 10.1016/s0169-5347(97)01048-3. [DOI] [PubMed] [Google Scholar]
- 28.Brian M V, Brian A D. Trans R Ent Soc Lond. 1952;103:1–26. [Google Scholar]
- 29.West Eberhard M J. Misc Publ Mus Zool Univ Michigan. 1969;140:1–101. [Google Scholar]
- 30.Smethurst, M. E. & Carpenter, J. M. (1998) J. N. Y. Entomol. Soc. 105, in press.
- 31.Jeanne R L. In: The Social Biology of Wasps. Ross K G, Matthews R W, editors. Ithaca, NY: Cornell Univ. Press; 1991. pp. 389–425. [Google Scholar]
- 32.Robinson G E. Annu Rev Entomol. 1992;37:637–665. doi: 10.1146/annurev.en.37.010192.003225. [DOI] [PubMed] [Google Scholar]
- 33.Matsuura M. In: The Social Biology of Wasps. Ross K G, Matthews R W, editors. Ithaca, NY: Cornell Univ. Press; 1991. pp. 232–262. [Google Scholar]
- 34.Greene A. In: The Social Biology of Wasps. Ross K G, Matthews R W, editors. Ithaca, NY: Cornell Univ. Press; 1991. pp. 263–305. [Google Scholar]
- 35.Downing H, Jeanne R L. J Ethol. 1987;5:63–66. [Google Scholar]
- 36.Jeanne R L. Bull Mus Comp Zool Harvard Univ. 1972;144:63–150. [Google Scholar]
- 37.Post D C, Jeanne R L, Erickson E H. In: Interindividual Behavioral Variability in Social Insects. Jeanne R L, editor. Boulder, CO: Westview Press; 1988. pp. 283–321. [Google Scholar]
- 38.Deleurance E P. Ann Sci Nat Zool. 1957;11:93–228. [Google Scholar]
- 39.O’Donnell S. Anim Behav. 1998;55:417–426. doi: 10.1006/anbe.1997.0627. [DOI] [PubMed] [Google Scholar]
- 40.Downing H, Jeanne R L. Anim Behav. 1990;39:105–124. [Google Scholar]
- 41.Karsai, I. & Pénzes, Z. (1998) Proc. R. Soc. London, in press.