Abstract
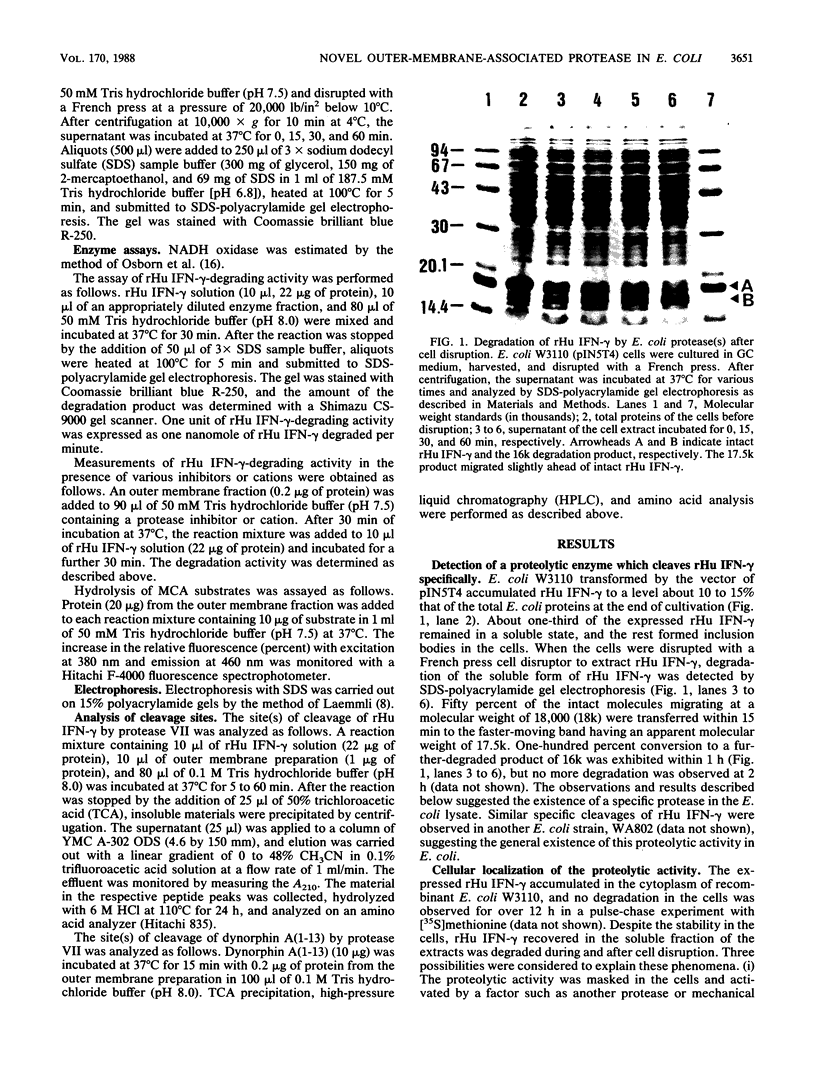
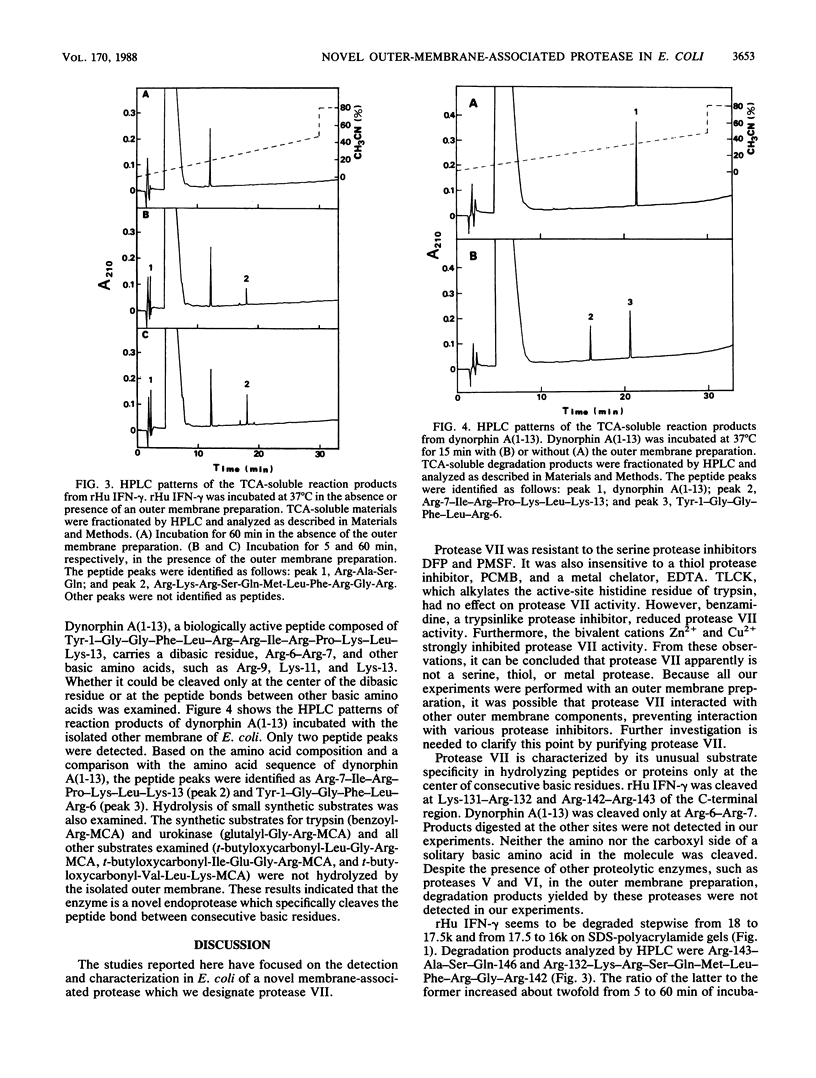
Human gamma interferon produced by recombinant Escherichia coli was degraded by endogenous protease after cell disruption. Specific cleavages took place at the center of two pairs of basic amino acids (Lys-131-Arg-132 and Arg-142-Arg-143) in the C-terminal region, giving rise to products with molecular weights of 17,500 and 16,000. The proteolytic activity was associated with the outer membrane of E. coli. It was insensitive to the protease inhibitors diisopropylfluorophosphate, phenylmethylsulfonyl fluoride, tosyl-L-lysine chloro-methyl ketone, EDTA, and p-chloromercuribenzoate. Benzamidine and the bivalent cations Zn2+ and Cu2+ inhibited the activity. Dynorphin A(1-13) (Tyr-Gly-Gly-Phe-Leu-Arg-Arg-Ile-Arg-Pro-Lys-Leu-Lys) was a good substrate and was preferentially cleaved at the center of Arg-6-Arg-7. Neither the amino nor carboxyl sides of Arg-9 and Lys-11 were digested. These results indicate that the protease specifically cleaves the peptide bond between consecutive basic residues and therefore is different from the known membrane enzymes, proteases IV, V, and VI. We have designated this new enzyme protease VII.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chung C. H., Ives H. E., Almeda S., Goldberg A. L. Purification from Escherichia coli of a periplasmic protein that is a potent inhibitor of pancreatic proteases. J Biol Chem. 1983 Sep 25;258(18):11032–11038. [PubMed] [Google Scholar]
- Gordon G., Gayda R. C., Markovitz A. Sequence of the regulatory region of omp T, the gene specifying major outer membrane protein a (3b) of Escherichia coli K-12: implications for regulation and processing. Mol Gen Genet. 1984;193(3):414–421. doi: 10.1007/BF00382077. [DOI] [PubMed] [Google Scholar]
- Gray P. W., Leung D. W., Pennica D., Yelverton E., Najarian R., Simonsen C. C., Derynck R., Sherwood P. J., Wallace D. M., Berger S. L. Expression of human immune interferon cDNA in E. coli and monkey cells. Nature. 1982 Feb 11;295(5849):503–508. doi: 10.1038/295503a0. [DOI] [PubMed] [Google Scholar]
- Hollifield W. C., Jr, Fiss E. H., Neilands J. B. Modification of a ferric enterobactin receptor protein from the outer membrane of Escherichia coli. Biochem Biophys Res Commun. 1978 Jul 28;83(2):739–746. doi: 10.1016/0006-291x(78)91051-3. [DOI] [PubMed] [Google Scholar]
- Ichihara S., Beppu N., Mizushima S. Protease IV, a cytoplasmic membrane protein of Escherichia coli, has signal peptide peptidase activity. J Biol Chem. 1984 Aug 10;259(15):9853–9857. [PubMed] [Google Scholar]
- Julius D., Brake A., Blair L., Kunisawa R., Thorner J. Isolation of the putative structural gene for the lysine-arginine-cleaving endopeptidase required for processing of yeast prepro-alpha-factor. Cell. 1984 Jul;37(3):1075–1089. doi: 10.1016/0092-8674(84)90442-2. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leytus S. P., Bowles L. K., Konisky J., Mangel W. F. Activation of plasminogen to plasmin by a protease associated with the outer membrane of Escherichia coli. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1485–1489. doi: 10.1073/pnas.78.3.1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuno K., Matsuo H. A novel protease from yeast with specificity towards paired basic residues. Nature. 1984 Jun 7;309(5968):558–560. doi: 10.1038/309558a0. [DOI] [PubMed] [Google Scholar]
- Mizuno K., Nakamura T., Takada K., Sakakibara S., Matsuo H. A membrane-bound, calcium-dependent protease in yeast alpha-cell cleaving on the carboxyl side of paired basic residues. Biochem Biophys Res Commun. 1987 Apr 29;144(2):807–814. doi: 10.1016/s0006-291x(87)80036-0. [DOI] [PubMed] [Google Scholar]
- Nakamura K., Inouye M. Construction of versatile expression cloning vehicles using the lipoprotein gene of Escherichia coli. EMBO J. 1982;1(6):771–775. doi: 10.1002/j.1460-2075.1982.tb01244.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborn M. J., Gander J. E., Parisi E., Carson J. Mechanism of assembly of the outer membrane of Salmonella typhimurium. Isolation and characterization of cytoplasmic and outer membrane. J Biol Chem. 1972 Jun 25;247(12):3962–3972. [PubMed] [Google Scholar]
- Pacaud M. Purification and characterization of two novel proteolytic enzymes in membranes of Escherichia coli. Protease IV and protease V. J Biol Chem. 1982 Apr 25;257(8):4333–4339. [PubMed] [Google Scholar]
- Pacaud M., Richaud C. Protease II from Escherichia coli. Purification and characterization. J Biol Chem. 1975 Oct 10;250(19):7771–7779. [PubMed] [Google Scholar]
- Palmer S. M., St John A. C. Characterization of a membrane-associated serine protease in Escherichia coli. J Bacteriol. 1987 Apr;169(4):1474–1479. doi: 10.1128/jb.169.4.1474-1479.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Régnier P. The purification of protease IV of E. coli and the demonstration that it is an endoproteolytic enzyme. Biochem Biophys Res Commun. 1981 Apr 30;99(4):1369–1376. doi: 10.1016/0006-291x(81)90770-1. [DOI] [PubMed] [Google Scholar]