Abstract
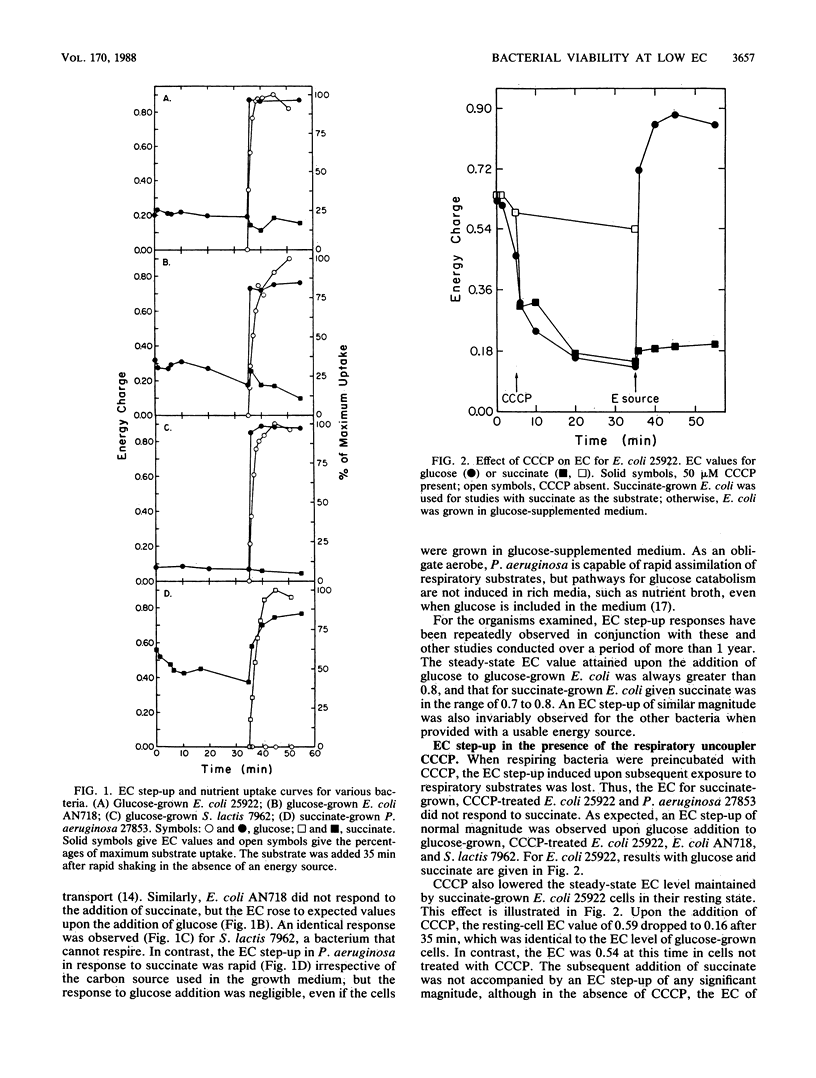
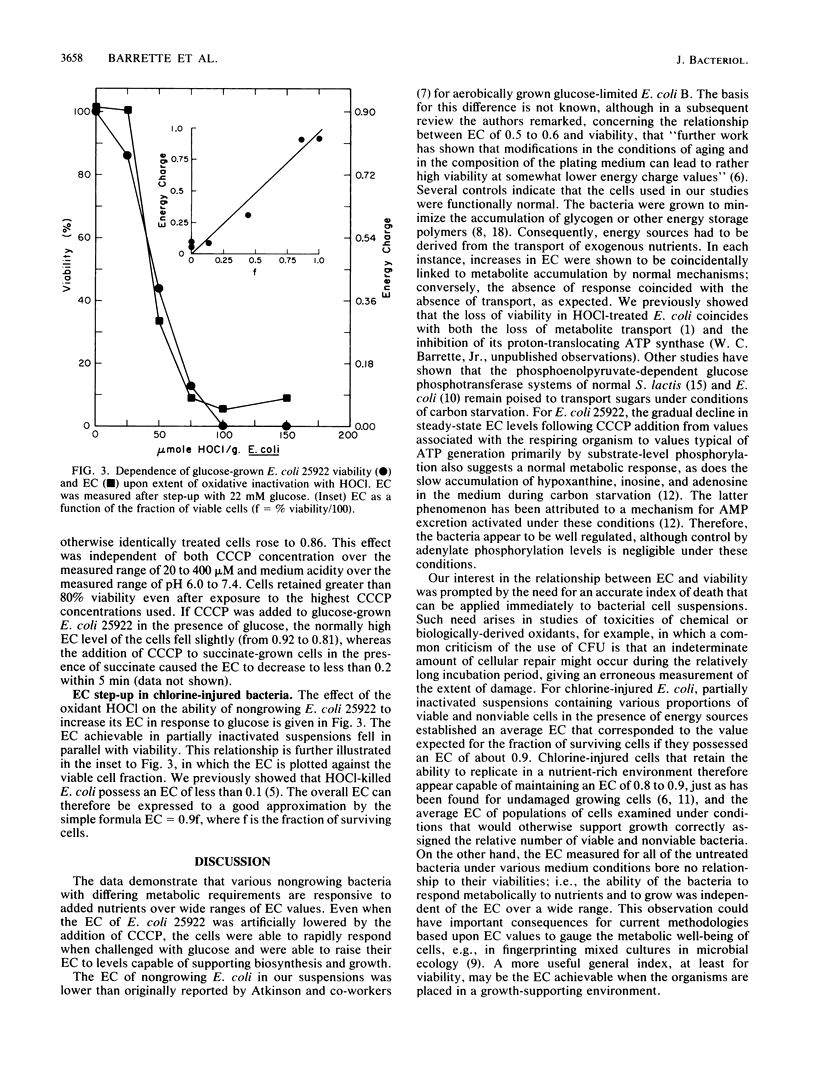
Metabolic regulation by nucleotides has been examined in several bacteria within the context of the adenylate energy charge (EC) concept. The ECs of bacteria capable of only fermentative metabolism (Streptococcus lactis and the ATPase-less mutant Escherichia coli AN718) fell to less than 0.2 under carbon-limiting conditions, but the bacteria were able to step up the EC to greater than 0.8 upon exposure to nutrient sugars. Similarly, nongrowing E. coli 25922, whose EC had been artificially lowered to less than 0.1 by the addition of the protonophore carbonyl cyanide m-chlorophenylhydrazone (CCCP), was able to immediately step up the EC to 0.8 to 0.9 upon the addition of glucose but was unable to respond to respiratory substrates. The EC of respiring bacteria (E. coli 25922 and Pseudomonas aeruginosa 27853) fell to 0.3 to 0.4 under certain limiting growth conditions, but the bacteria also responded immediately when challenged with succinate to give EC values greater than 0.8. These bacteria could not step up the EC with respiratory substrates in the presence of CCCP. For all bacteria, the loss of the ability to step up the EC was attributable to the loss of nutrient transport function. Mixtures of viable and HOCl-killed E. coli 25922 were able to step up the EC in proportion to the fraction of surviving cells. The data indicate that nucleotide phosphorylation levels are not regulatory in nongrowing bacteria but that the EC step-up achievable upon nutrient addition may be an accurate index of viability.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albrich J. M., Gilbaugh J. H., 3rd, Callahan K. B., Hurst J. K. Effects of the putative neutrophil-generated toxin, hypochlorous acid, on membrane permeability and transport systems of Escherichia coli. J Clin Invest. 1986 Jul;78(1):177–184. doi: 10.1172/JCI112548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albrich J. M., Hurst J. K. Oxidative inactivation of Escherichia coli by hypochlorous acid. Rates and differentiation of respiratory from other reaction sites. FEBS Lett. 1982 Jul 19;144(1):157–161. doi: 10.1016/0014-5793(82)80591-7. [DOI] [PubMed] [Google Scholar]
- Atkinson D. E. The energy charge of the adenylate pool as a regulatory parameter. Interaction with feedback modifiers. Biochemistry. 1968 Nov;7(11):4030–4034. doi: 10.1021/bi00851a033. [DOI] [PubMed] [Google Scholar]
- Ball W. J., Jr, Atkinson D. E. Adenylate energy charge in Saccharomyces cerevisiae during starvation. J Bacteriol. 1975 Mar;121(3):975–982. doi: 10.1128/jb.121.3.975-982.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrette W. C., Jr, Albrich J. M., Hurst J. K. Hypochlorous acid-promoted loss of metabolic energy in Escherichia coli. Infect Immun. 1987 Oct;55(10):2518–2525. doi: 10.1128/iai.55.10.2518-2525.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman A. G., Atkinson D. E. Adenine nucleotide concentrations and turnover rates. Their correlation with biological activity in bacteria and yeast. Adv Microb Physiol. 1977;15:253–306. doi: 10.1016/s0065-2911(08)60318-5. [DOI] [PubMed] [Google Scholar]
- Chapman A. G., Fall L., Atkinson D. E. Adenylate energy charge in Escherichia coli during growth and starvation. J Bacteriol. 1971 Dec;108(3):1072–1086. doi: 10.1128/jb.108.3.1072-1086.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karl D. M. Cellular nucleotide measurements and applications in microbial ecology. Microbiol Rev. 1980 Dec;44(4):739–796. doi: 10.1128/mr.44.4.739-796.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein W. L., Boyer P. D. Energization of active transport by Escherichia coli. J Biol Chem. 1972 Nov 25;247(22):7257–7265. [PubMed] [Google Scholar]
- Leung H. B., Schramm V. L. Adenylate degradation in Escherichia coli. The role of AMP nucleosidase and properties of the purified enzyme. J Biol Chem. 1980 Nov 25;255(22):10867–10874. [PubMed] [Google Scholar]
- Lo T. C., Rayman M. K., Sanwal B. D. Transport of succinate in Escherichia coli. I. Biochemical and genetic studies of transport in whole cells. J Biol Chem. 1972 Oct 10;247(19):6323–6331. [PubMed] [Google Scholar]
- Mason P. W., Carbone D. P., Cushman R. A., Waggoner A. S. The importance of inorganic phosphate in regulation of energy metabolism of Streptococcus lactis. J Biol Chem. 1981 Feb 25;256(4):1861–1866. [PubMed] [Google Scholar]
- Phibbs P. V., Jr, Eagon R. G. Transport and phosphorylation of glucose, fructose, and mannitol by Pseudomonas aeruginosa. Arch Biochem Biophys. 1970 Jun;138(2):470–482. doi: 10.1016/0003-9861(70)90371-1. [DOI] [PubMed] [Google Scholar]
- Preiss J. Bacterial glycogen synthesis and its regulation. Annu Rev Microbiol. 1984;38:419–458. doi: 10.1146/annurev.mi.38.100184.002223. [DOI] [PubMed] [Google Scholar]
- Williams J. C., Weiss E. Energy metabolism of Rickettsia typhi: pools of adenine nucleotides and energy charge in the presence and absence of glutamate. J Bacteriol. 1978 Jun;134(3):884–892. doi: 10.1128/jb.134.3.884-892.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]



