Abstract
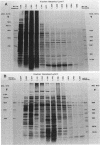
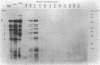
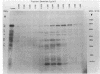
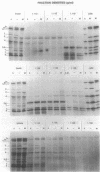
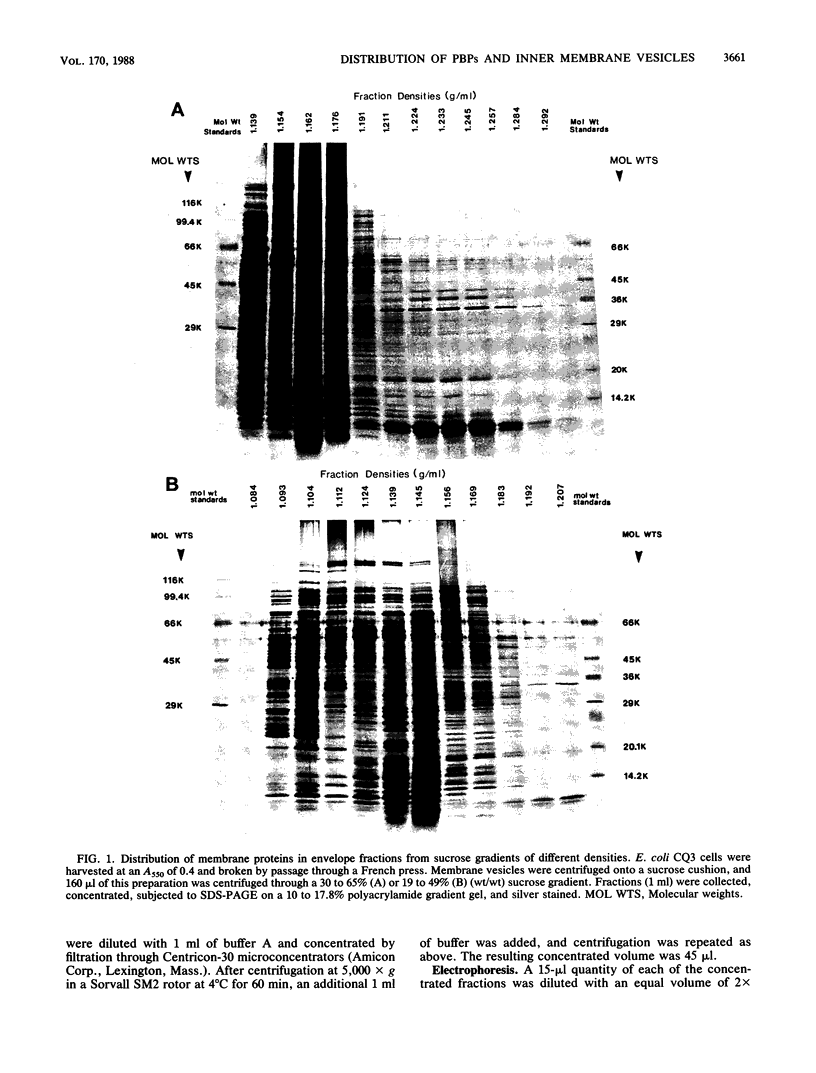
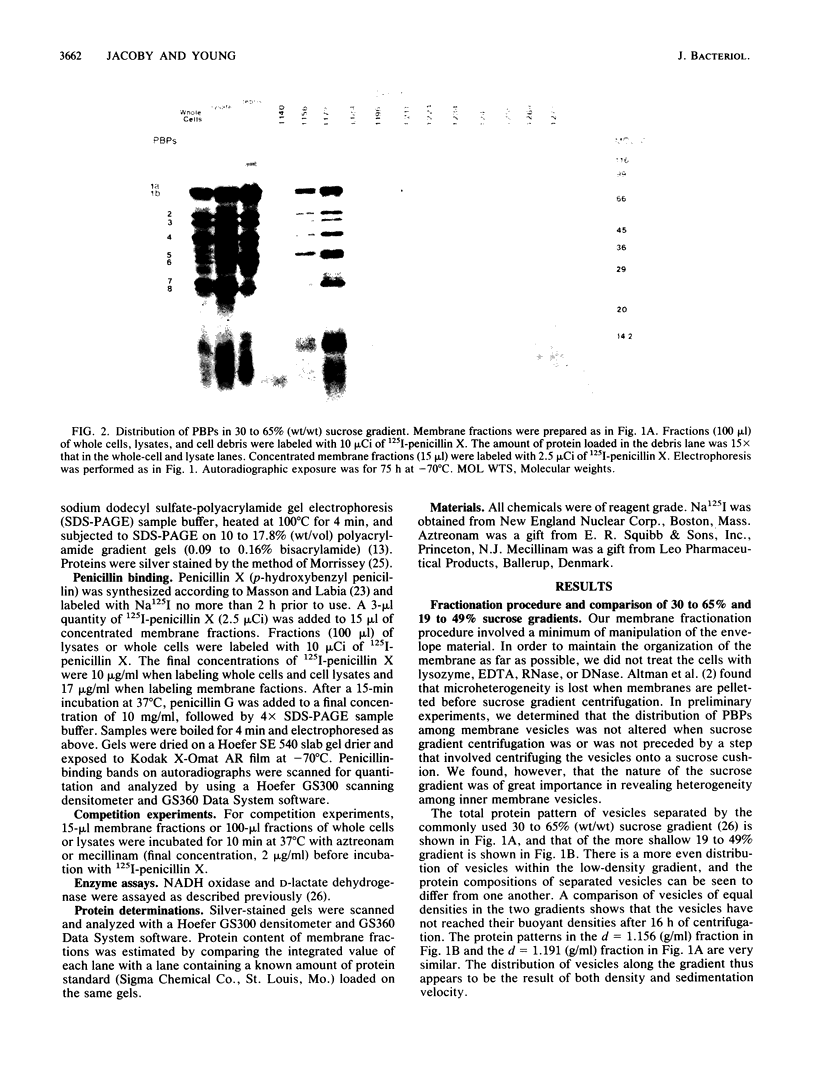
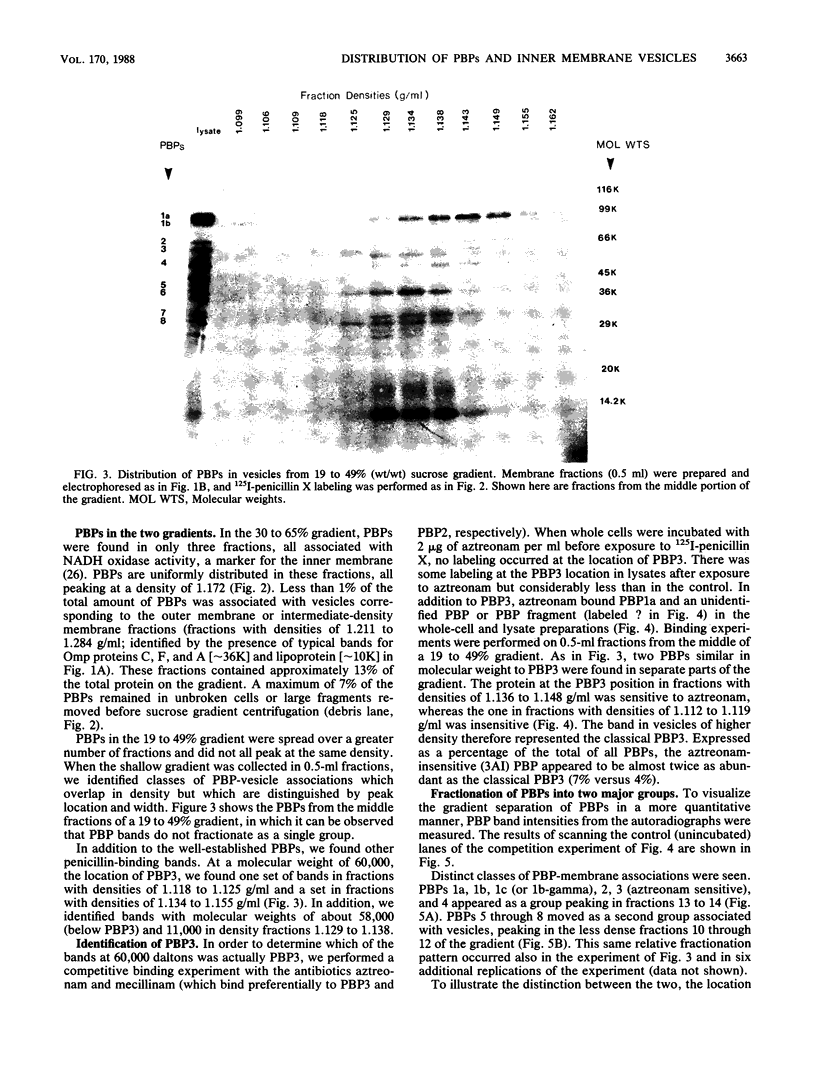
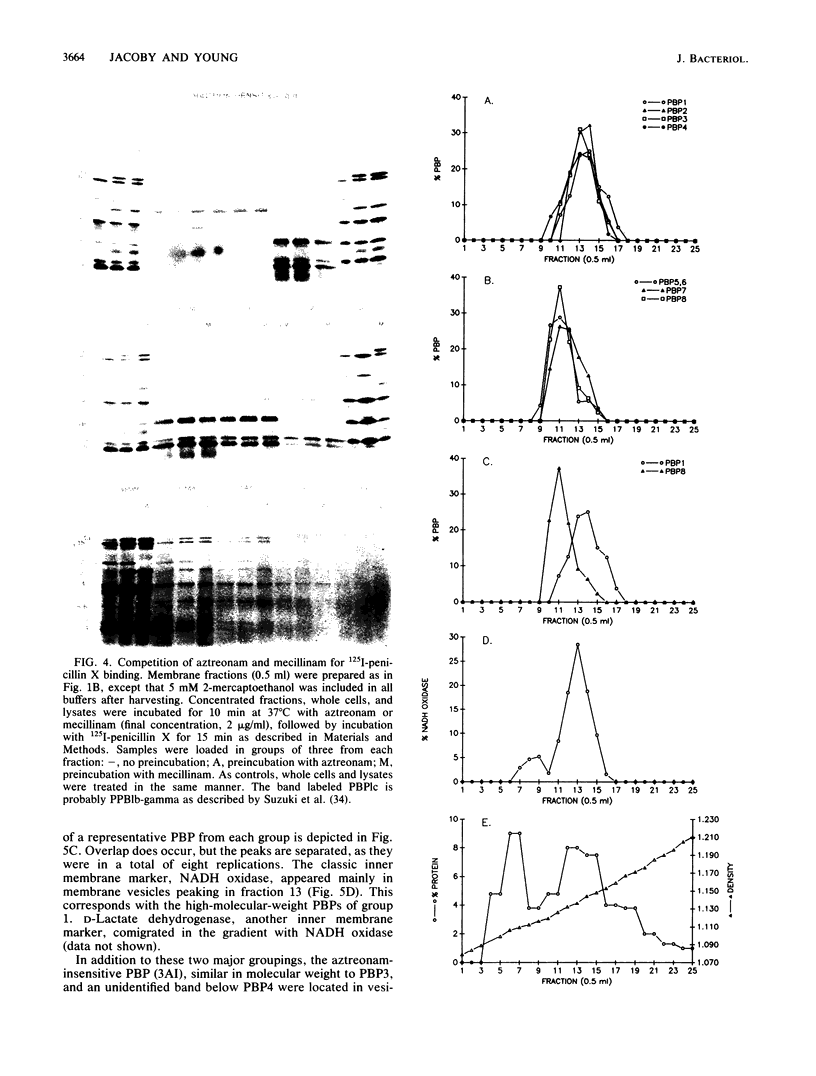
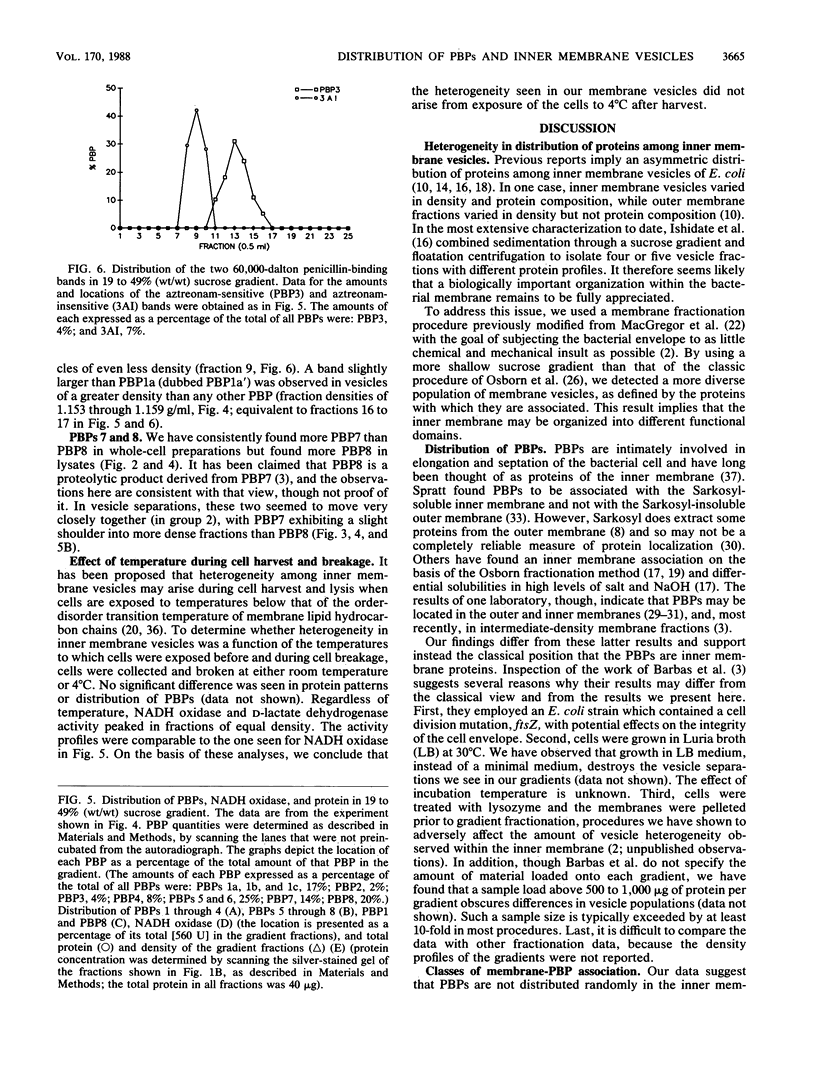
Escherichia coli penicillin-binding proteins (PBPs) were associated only with inner membrane vesicles when separated on 30 to 65% or 19 to 49% (wt/wt) sucrose gradients. Fractionation of vesicles through the low-density gradient revealed at least two classes of PBP-inner membrane associations. The first class consisted of PBPs 1 through 4, and the second class consisted of PBPs 5 through 8. These classes were distinguished by the density of vesicles with which they were associated; class 1 PBPs migrated with vesicles of higher density than did class 2 PBPs. Such combinations suggest that PBPs are nonrandomly distributed within the inner membrane, implying potential functional relationships among the PBPs themselves and with particular membrane domains. In addition, in cell lysates and in vesicle fractions, a 60,000-dalton aztreonam-insensitive PBP or protein fragment was observed which could potentially be confused with PBP3.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altman E., Altman R. K., Garrett J. M., Grimaila R. J., Young R. S gene product: identification and membrane localization of a lysis control protein. J Bacteriol. 1983 Sep;155(3):1130–1137. doi: 10.1128/jb.155.3.1130-1137.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altman E., Young K., Garrett J., Altman R., Young R. Subcellular localization of lethal lysis proteins of bacteriophages lambda and phiX174. J Virol. 1985 Mar;53(3):1008–1011. doi: 10.1128/jvi.53.3.1008-1011.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbas J. A., Díaz J., Rodríguez-Tébar A., Vázquez D. Specific location of penicillin-binding proteins within the cell envelope of Escherichia coli. J Bacteriol. 1986 Jan;165(1):269–275. doi: 10.1128/jb.165.1.269-275.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer M. H., Costello G. P., Bayer M. E. Isolation and partial characterization of membrane vesicles carrying markers of the membrane adhesion sites. J Bacteriol. 1982 Feb;149(2):758–767. doi: 10.1128/jb.149.2.758-767.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bone E. J., Todd J. A., Ellar D. J., Sargent M. G., Wyke A. W. Membrane particles from Escherichia coli and Bacillus subtilis, containing penicillin-binding proteins and enriched for chromosomal-origin DNA. J Bacteriol. 1985 Oct;164(1):192–200. doi: 10.1128/jb.164.1.192-200.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botta G. A., Park J. T. Evidence for involvement of penicillin-binding protein 3 in murein synthesis during septation but not during cell elongation. J Bacteriol. 1981 Jan;145(1):333–340. doi: 10.1128/jb.145.1.333-340.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caulfield M. P., Tai P. C., Davis B. D. Association of penicillin-binding proteins and other enzymes with the ribosome-free membrane fraction of Bacillus subtilis. J Bacteriol. 1983 Oct;156(1):1–5. doi: 10.1128/jb.156.1.1-5.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chopra I., Shales S. W. Comparison of the polypeptide composition of Escherichia coli outer membranes prepared by two methods. J Bacteriol. 1980 Oct;144(1):425–427. doi: 10.1128/jb.144.1.425-427.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Leij L., Witholt B. Structural heterogeneity of the cytoplasmic and outer membranes of Escherichia coli. Biochim Biophys Acta. 1977 Nov 15;471(1):92–104. doi: 10.1016/0005-2736(77)90396-0. [DOI] [PubMed] [Google Scholar]
- Descoteaux A., Drapeau G. R. Regulation of cell division in Escherichia coli K-12: probable interactions among proteins FtsQ, FtsA, and FtsZ. J Bacteriol. 1987 May;169(5):1938–1942. doi: 10.1128/jb.169.5.1938-1942.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dopazo A., Tormo A., Aldea M., Vicente M. Structural inhibition and reactivation of Escherichia coli septation by elements of the SOS and TER pathways. J Bacteriol. 1987 Apr;169(4):1772–1776. doi: 10.1128/jb.169.4.1772-1776.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreyfuss G., Adam S. A., Choi Y. D. Physical change in cytoplasmic messenger ribonucleoproteins in cells treated with inhibitors of mRNA transcription. Mol Cell Biol. 1984 Mar;4(3):415–423. doi: 10.1128/mcb.4.3.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodell E. W., Schwarz U., Teather R. M. Cell envelope composition of Escherichia coli K12: a comparison of the cell poles and the lateral wall. Eur J Biochem. 1974 Sep 16;47(3):567–572. doi: 10.1111/j.1432-1033.1974.tb03727.x. [DOI] [PubMed] [Google Scholar]
- Guan T., Ghosh A., Ghosh B. K. Immunoelectron microscopic double labeling of alkaline phosphatase and penicillinase with colloidal gold in frozen thin sections of Bacillus licheniformis 749/C. J Bacteriol. 1985 Oct;164(1):107–113. doi: 10.1128/jb.164.1.107-113.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishidate K., Creeger E. S., Zrike J., Deb S., Glauner B., MacAlister T. J., Rothfield L. I. Isolation of differentiated membrane domains from Escherichia coli and Salmonella typhimurium, including a fraction containing attachment sites between the inner and outer membranes and the murein skeleton of the cell envelope. J Biol Chem. 1986 Jan 5;261(1):428–443. [PubMed] [Google Scholar]
- Jackson M. E., Pratt J. M., Stoker N. G., Holland I. B. An inner membrane protein N-terminal signal sequence is able to promote efficient localisation of an outer membrane protein in Escherichia coli. EMBO J. 1985 Sep;4(9):2377–2383. doi: 10.1002/j.1460-2075.1985.tb03942.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseleau-Petit D., Kepes A. A novel electrophoretic fractionation of Escherichia coli envelopes. Biochim Biophys Acta. 1975 Sep 16;406(1):36–49. doi: 10.1016/0005-2736(75)90040-1. [DOI] [PubMed] [Google Scholar]
- Koyasu S., Fukuda A., Okada Y. The penicillin-binding proteins of Caulobacter crescentus. J Biochem. 1980 Jan;87(1):363–366. doi: 10.1093/oxfordjournals.jbchem.a132749. [DOI] [PubMed] [Google Scholar]
- Letellier L., Moudden H., Shechter E. Lipid and protein segregation in Escherichia coli membrane: morphological and structural study of different cytoplasmic membrane fractions. Proc Natl Acad Sci U S A. 1977 Feb;74(2):452–456. doi: 10.1073/pnas.74.2.452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacGregor C. H., Bishop C. W., Blech J. E. Localization of proteolytic activity in the outer membrane of Escherichia coli. J Bacteriol. 1979 Jan;137(1):574–583. doi: 10.1128/jb.137.1.574-583.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macalister T. J., Macdonald B., Rothfield L. I. The periseptal annulus: An organelle associated with cell division in Gram-negative bacteria. Proc Natl Acad Sci U S A. 1983 Mar;80(5):1372–1376. doi: 10.1073/pnas.80.5.1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masson J. M., Labia R. Synthesis of a 125I-radiolabeled penicillin for penicillin-binding proteins studies. Anal Biochem. 1983 Jan;128(1):164–168. doi: 10.1016/0003-2697(83)90357-3. [DOI] [PubMed] [Google Scholar]
- Morrissey J. H. Silver stain for proteins in polyacrylamide gels: a modified procedure with enhanced uniform sensitivity. Anal Biochem. 1981 Nov 1;117(2):307–310. doi: 10.1016/0003-2697(81)90783-1. [DOI] [PubMed] [Google Scholar]
- Osborn M. J., Gander J. E., Parisi E., Carson J. Mechanism of assembly of the outer membrane of Salmonella typhimurium. Isolation and characterization of cytoplasmic and outer membrane. J Biol Chem. 1972 Jun 25;247(12):3962–3972. [PubMed] [Google Scholar]
- Pisabarro A. G., Prats R., Váquez D., Rodríguez-Tébar A. Activity of penicillin-binding protein 3 from Escherichia coli. J Bacteriol. 1986 Oct;168(1):199–206. doi: 10.1128/jb.168.1.199-206.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez-Tébar A., Barbas J. A., Vázquez D. Location of some proteins involved in peptidoglycan synthesis and cell division in the inner and outer membranes of Escherichia coli. J Bacteriol. 1985 Jan;161(1):243–248. doi: 10.1128/jb.161.1.243-248.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnaitman C. A. Protein composition of the cell wall and cytoplasmic membrane of Escherichia coli. J Bacteriol. 1970 Nov;104(2):890–901. doi: 10.1128/jb.104.2.890-901.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spratt B. G. Properties of the penicillin-binding proteins of Escherichia coli K12,. Eur J Biochem. 1977 Jan;72(2):341–352. doi: 10.1111/j.1432-1033.1977.tb11258.x. [DOI] [PubMed] [Google Scholar]
- Suzuki H., Kato J., Sakagami Y., Mori M., Suzuki A., Hirota Y. Conversion of the alpha component of penicillin-binding protein 1b to the beta component in Escherichia coli. J Bacteriol. 1987 Feb;169(2):891–893. doi: 10.1128/jb.169.2.891-893.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tormo A., Ayala J. A., de Pedro M. A., Aldea M., Vicente M. Interaction of FtsA and PBP3 proteins in the Escherichia coli septum. J Bacteriol. 1986 Jun;166(3):985–992. doi: 10.1128/jb.166.3.985-992.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waxman D. J., Strominger J. L. Penicillin-binding proteins and the mechanism of action of beta-lactam antibiotics. Annu Rev Biochem. 1983;52:825–869. doi: 10.1146/annurev.bi.52.070183.004141. [DOI] [PubMed] [Google Scholar]
- van Heerikhuizen H., Kwak E., van Bruggen E. F., Witholt B. Characterization of a low density cytoplasmic membrane subfraction isolated from Escherichia coli. Biochim Biophys Acta. 1975 Dec 1;413(2):177–191. doi: 10.1016/0005-2736(75)90102-9. [DOI] [PubMed] [Google Scholar]