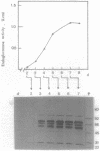
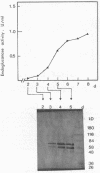
Abstract
A method consisting of sodium dodecyl sulfate-polyacrylamide gel electrophoresis and subsequent detection of endoglucanases by blotting with a polyclonal antibody against endoglucanase I was used to investigate the effect of induction and carbon catabolite derepression on the synthesis of multiple forms of endoglucanase I by Trichoderma reesei. Five forms appeared upon growth on cellulose, whereas four and only two appeared upon growth on lactose (carbon catabolite derepression) and induction by sophorose in a resting cell system, respectively. All endoglucanases detected resembled endoglucanase I in their specificity, since they exhibited no activity toward xylan or paranitrophenyl-beta-D-lactobioside. A small (25-kilodalton) endoglucanase only appeared during growth on cellulose. None of the multiple forms arose by postsecretional modification. The results indicate that sophorose may not be the only compound mediating cellulose induction of the specific endoglucanases in T. reesei.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beldman G., Searle-Van Leeuwen M. F., Rombouts F. M., Voragen F. G. The cellulase of Trichoderma viride. Purification, characterization and comparison of all detectable endoglucanases, exoglucanases and beta-glucosidases. Eur J Biochem. 1985 Jan 15;146(2):301–308. doi: 10.1111/j.1432-1033.1985.tb08653.x. [DOI] [PubMed] [Google Scholar]
- Biely P., Markovic O., Mislovicová D. Sensitive detection of endo-1,4-beta-glucanases and endo-1,4-beta-xylanases in gels. Anal Biochem. 1985 Jan;144(1):147–151. doi: 10.1016/0003-2697(85)90096-x. [DOI] [PubMed] [Google Scholar]
- Glenn M., Ghosh A., Ghosh B. K. Subcellular fractionation of a hypercellulolytic mutant, Trichoderma reesei Rut-C30: localization of endoglucanase in microsomal fraction. Appl Environ Microbiol. 1985 Nov;50(5):1137–1143. doi: 10.1128/aem.50.5.1137-1143.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Håkansson U., Fägerstam L. G., Pettersson L. G., Andersson L. A 1,4-beta-glucan glucanohydrolase from the cellulolytic fungus Trichoderma viride QM 9414. Purification, characterization and preparation of an immunoadsorbent for the enzyme. Biochem J. 1979 Apr 1;179(1):141–149. doi: 10.1042/bj1790141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Håkansson U., Fägerstam L., Pettersson G., Andersson L. Purification and characterization of a low molecular weight 1,4-beta-glucan glucanohydrolase from the cellulolytic fungus Trichoderma viride QM 9414. Biochim Biophys Acta. 1978 Jun 9;524(2):385–392. doi: 10.1016/0005-2744(78)90175-4. [DOI] [PubMed] [Google Scholar]
- Kubicek C. P. Involvement of a conidial endoglucanase and a plasma-membrane-bound beta-glucosidase in the induction of endoglucanase synthesis by cellulose in Trichoderma reesei. J Gen Microbiol. 1987 Jun;133(6):1481–1487. doi: 10.1099/00221287-133-6-1481. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- MANDELS M., PARRISH F. W., REESE E. T. Sophorose as an inducer of cellulase in Trichoderma viride. J Bacteriol. 1962 Feb;83:400–408. doi: 10.1128/jb.83.2.400-408.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merivuori H., Siegler K. M., Sands J. A., Montenecourt B. S. Regulation of cellulase biosynthesis and secretion in fungi. Biochem Soc Trans. 1985 Apr;13(2):411–414. doi: 10.1042/bst0130411. [DOI] [PubMed] [Google Scholar]
- Montenecourt B. S., Nhlapo S. D., Trimiño-Vazquez H., Cuskey S., Schamhart D. H., Eveleigh D. E. Regulatory controls in relation to over-production of fungal cellulases. Basic Life Sci. 1981;18:33–53. doi: 10.1007/978-1-4684-3980-9_4. [DOI] [PubMed] [Google Scholar]
- Nakayama M., Tomita Y., Suzuki H., Nisizawa K. Partial proteolysis of some cellulase components from Trichoderma viride and the substrate specificity of the modified products. J Biochem. 1976 May;79(5):955–966. doi: 10.1093/oxfordjournals.jbchem.a131163. [DOI] [PubMed] [Google Scholar]
- Niku-Paavola M. L., Lappalainen A., Enari T. M., Nummi M. A new appraisal of the endoglucanases of the fungus Trichoderma reesei. Biochem J. 1985 Oct 1;231(1):75–81. doi: 10.1042/bj2310075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nisizawa T., Suzuki H., Nakayama M., Nisizawa K. Inductive formation of cellulase by sophorose in Trichoderma viride. J Biochem. 1971 Sep;70(3):375–385. doi: 10.1093/oxfordjournals.jbchem.a129652. [DOI] [PubMed] [Google Scholar]
- Nisizawa T., Suzuki H., Nisizawa K. Catabolite repression of cellulase formation in Trichoderma viride. J Biochem. 1972 Jun;71(6):999–1007. doi: 10.1093/oxfordjournals.jbchem.a129872. [DOI] [PubMed] [Google Scholar]
- Penttilä M., Lehtovaara P., Nevalainen H., Bhikhabhai R., Knowles J. Homology between cellulase genes of Trichoderma reesei: complete nucleotide sequence of the endoglucanase I gene. Gene. 1986;45(3):253–263. doi: 10.1016/0378-1119(86)90023-5. [DOI] [PubMed] [Google Scholar]
- Shoemaker S. P., Brown R. D., Jr Characterization of endo-1,4-beta-D-glucanases purified from Trichoderma viride. Biochim Biophys Acta. 1978 Mar 14;523(1):147–161. doi: 10.1016/0005-2744(78)90017-7. [DOI] [PubMed] [Google Scholar]
- Shoemaker S. P., Raymond J. C., Bruner R. Cellulases: diversity amongst improved Trichoderma strains. Basic Life Sci. 1981;18:89–109. doi: 10.1007/978-1-4684-3980-9_7. [DOI] [PubMed] [Google Scholar]
- Sternberg D., Mandels G. R. Induction of cellulolytic enzymes in Trichoderma reesei by sophorose. J Bacteriol. 1979 Sep;139(3):761–769. doi: 10.1128/jb.139.3.761-769.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]