Abstract
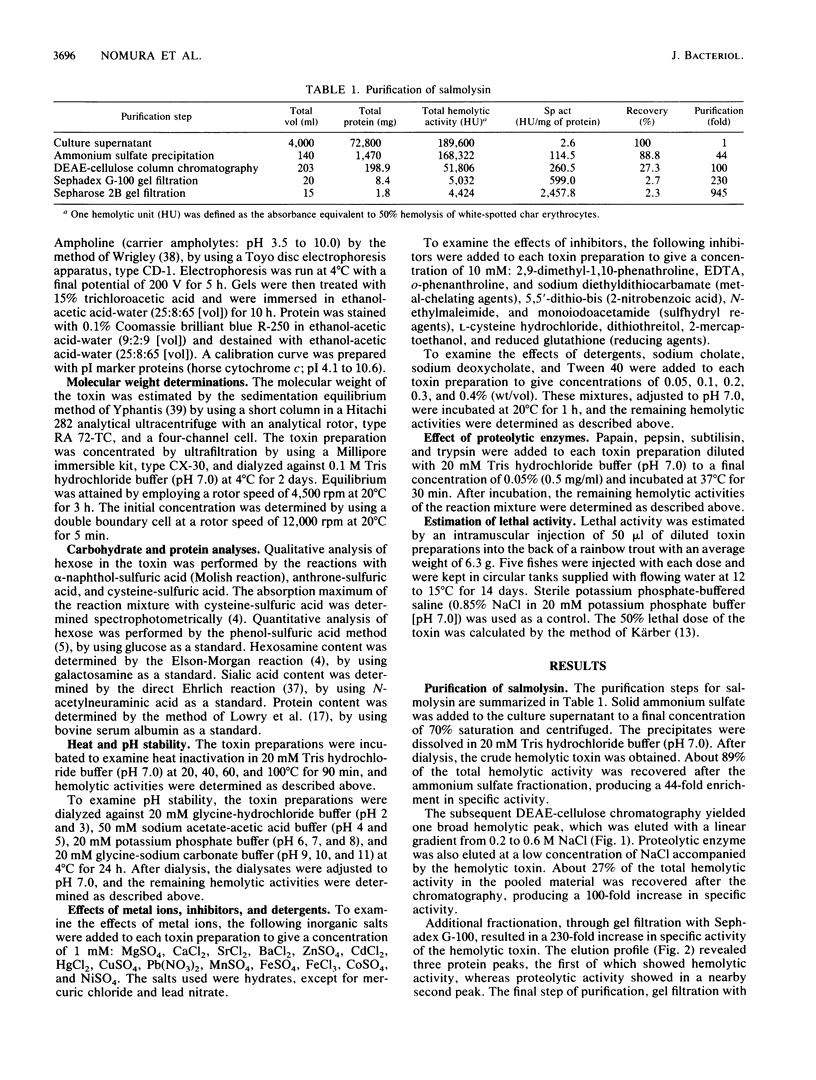
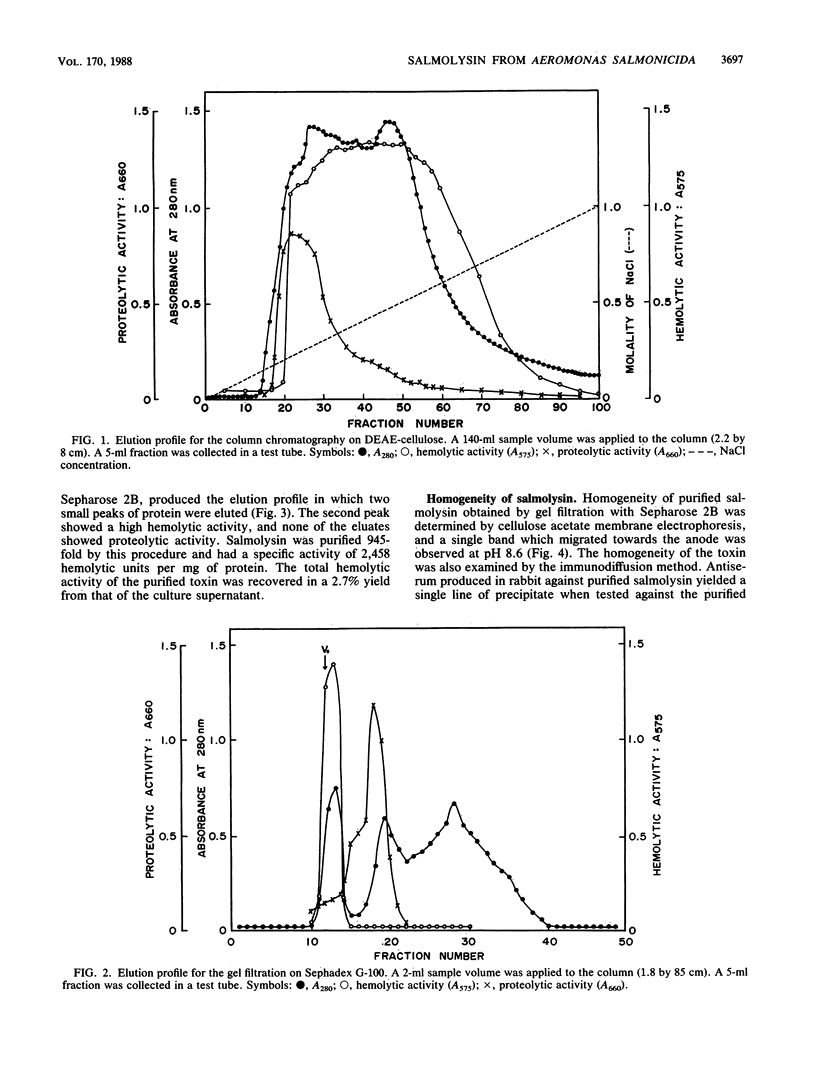
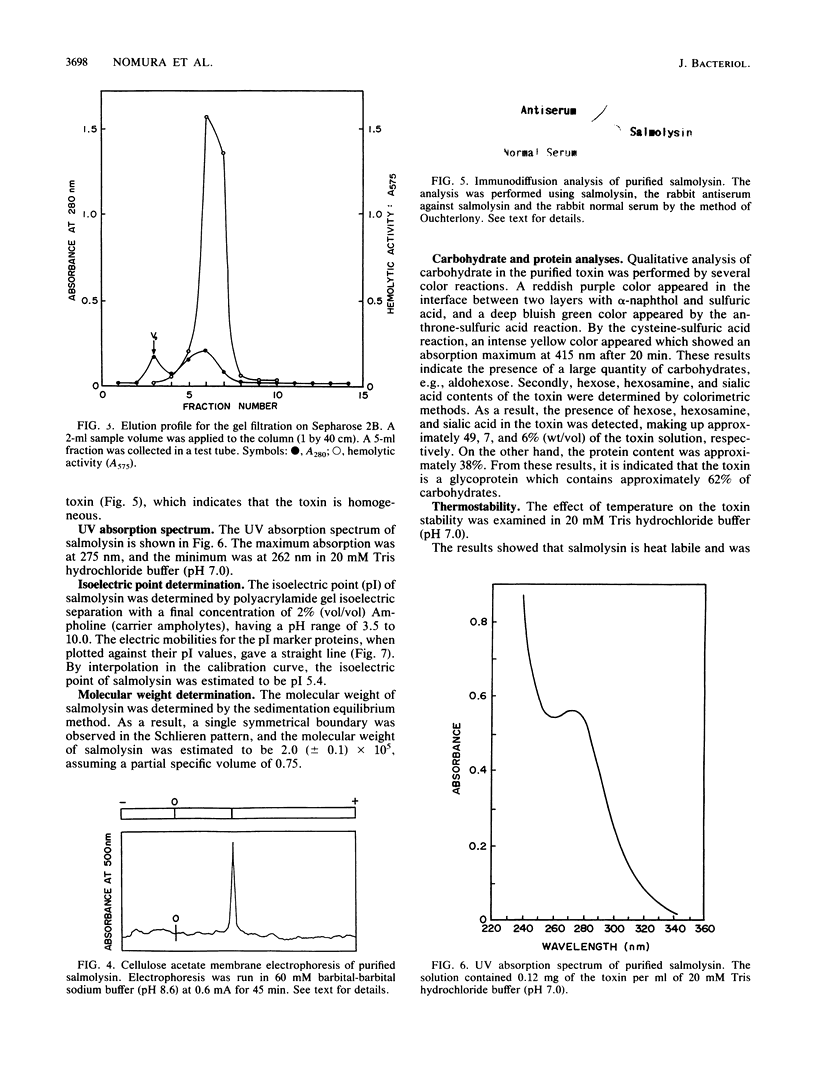
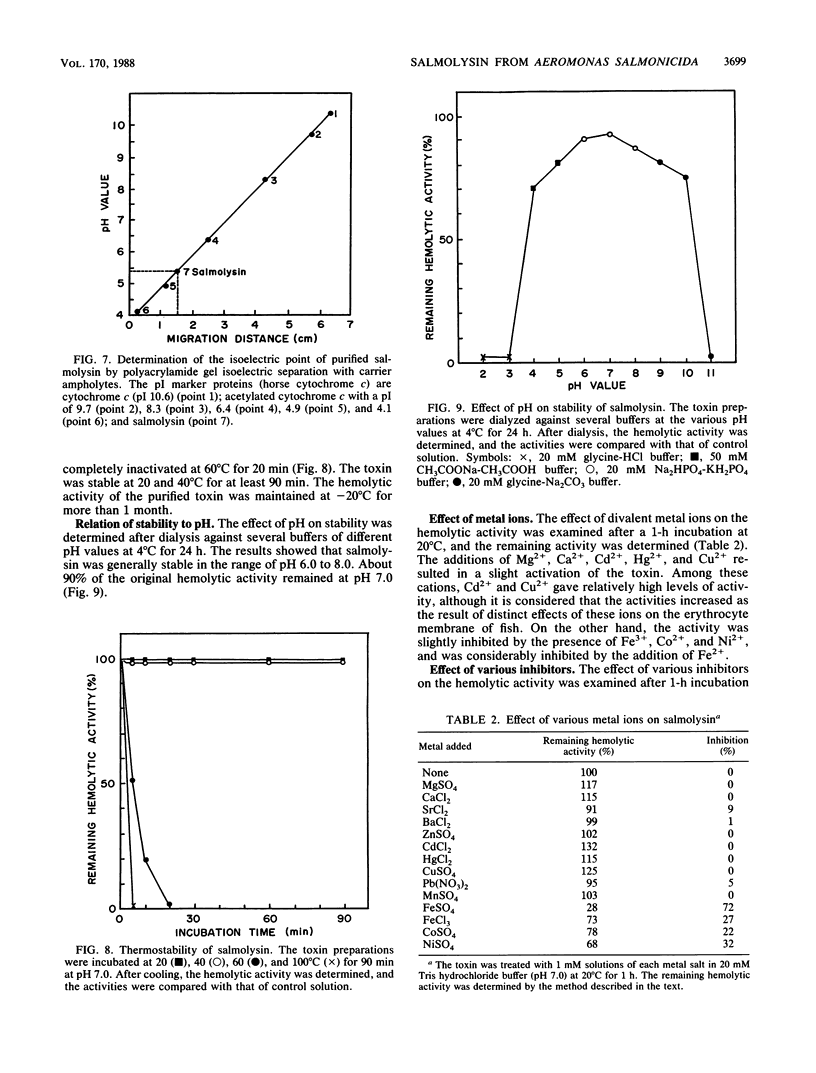
An extracellular hemolytic toxin of Aeromonas salmonicida, termed salmolysin, was purified 945-fold by ammonium sulfate precipitation, anion-exchange chromatography on DEAE-cellulose, and gel filtration chromatography on Sephadex G-100 and Sepharose 2B. Salmolysin appeared homogeneous upon cellulose acetate membrane electrophoresis and immunodiffusion analysis. The molecular weight of the toxin was estimated to be approximately 200,000 by the sedimentation equilibrium method. The UV absorption spectrum showed a maximum at 275 nm and a minimum at 262 nm. The isoelectric point was found to be at pI 5.4. Carbohydrate and protein analyses and other biochemical data indicated that salmolysin is a glycoprotein, containing approximately 62% carbohydrates. The toxin is a heat-labile substance and is stable at a neutral pH value. Ferrous ion inhibited the activity, whereas metal-chelating agents did not affect the activity. Sulfhydryl reagents did not inhibit the toxin, whereas reducing agents, such as L-cysteine and reduced glutathione, inhibited the toxin to a certain extent. Salmolysin was inactivated by a nonionic detergent but was stimulated by an anionic detergent, sodium deoxycholate, at a low concentration. The toxin was also inactivated by subtilisin and trypsin but was not inhibited by papain and pepsin. Salmolysin, with a remarkable hemolytic activity against salmonid erythrocytes, was lethal to rainbow trout when it was injected intramuscularly.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Buckley J. T., Halasa L. N., MacIntyre S. Purification and partial characterization of a bacterial phospholipid: cholesterol acyltransferase. J Biol Chem. 1982 Mar 25;257(6):3320–3325. [PubMed] [Google Scholar]
- Dahle H. K. The purification and some properties of two Aeromonas proteinases. Acta Pathol Microbiol Scand B Microbiol Immunol. 1971;79(6):726–738. doi: 10.1111/j.1699-0463.1971.tb00104.x. [DOI] [PubMed] [Google Scholar]
- Jenkins E. M., Watson B. B. Extracellular Antigens from Listeria monocytogenes I. Purification and Resolution of Hemolytic and Lipolytic Antigens from Culture Filtrates of Listeria monocytogenes. Infect Immun. 1971 Apr;3(4):589–594. doi: 10.1128/iai.3.4.589-594.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay W. W., Buckley J. T., Ishiguro E. E., Phipps B. M., Monette J. P., Trust T. J. Purification and disposition of a surface protein associated with virulence of Aeromonas salmonicida. J Bacteriol. 1981 Sep;147(3):1077–1084. doi: 10.1128/jb.147.3.1077-1084.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MacIntyre S., Trust T. J., Buckley J. T. Distribution of glycerophospholipid-cholesterol acyltransferase in selected bacterial species. J Bacteriol. 1979 Jul;139(1):132–136. doi: 10.1128/jb.139.1.132-136.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchlewicz B. A., Duncan J. L. Properties of a hemolysin produced by group B streptococci. Infect Immun. 1980 Dec;30(3):805–813. doi: 10.1128/iai.30.3.805-813.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OUCHTERLONY O. Antigen-antibody reactions in gels. IV. Types of reactions in coordinated systems of diffusion. Acta Pathol Microbiol Scand. 1953;32(2):230–240. [PubMed] [Google Scholar]
- Schenkein I., Green R. F., Santos D. S., Maas W. K. Partial purification and characterization of a heat-labile enterotoxin of Escherichia coli. Infect Immun. 1976 Jun;13(6):1710–1720. doi: 10.1128/iai.13.6.1710-1720.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi S., Azuma R., Suto T. Purification and some properties of hemolysin produced by Corynebacterium pyogenes. Nihon Juigaku Zasshi. 1979 Oct;41(5):511–516. doi: 10.1292/jvms1939.41.511. [DOI] [PubMed] [Google Scholar]
- Titball R. W., Bell A., Munn C. B. Role of caseinase from Aeromonas salmonicida in activation of hemolysin. Infect Immun. 1985 Sep;49(3):756–759. doi: 10.1128/iai.49.3.756-759.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Titball R. W., Munn C. B. The purification and some properties of H-lysin from Aeromonas salmonicida. J Gen Microbiol. 1985 Jul;131(7):1603–1609. doi: 10.1099/00221287-131-7-1603. [DOI] [PubMed] [Google Scholar]
- WERNER I., ODIN L. On the presence of sialic acid in certain glycoproteins and in gangliosides. Acta Soc Med Ups. 1952;57(3-4):230–241. [PubMed] [Google Scholar]
- YPHANSTIS D. A. Rapid determination of molecular weights of peptides and preteins. Ann N Y Acad Sci. 1960 Aug 31;88:586–601. doi: 10.1111/j.1749-6632.1960.tb20055.x. [DOI] [PubMed] [Google Scholar]




