Abstract
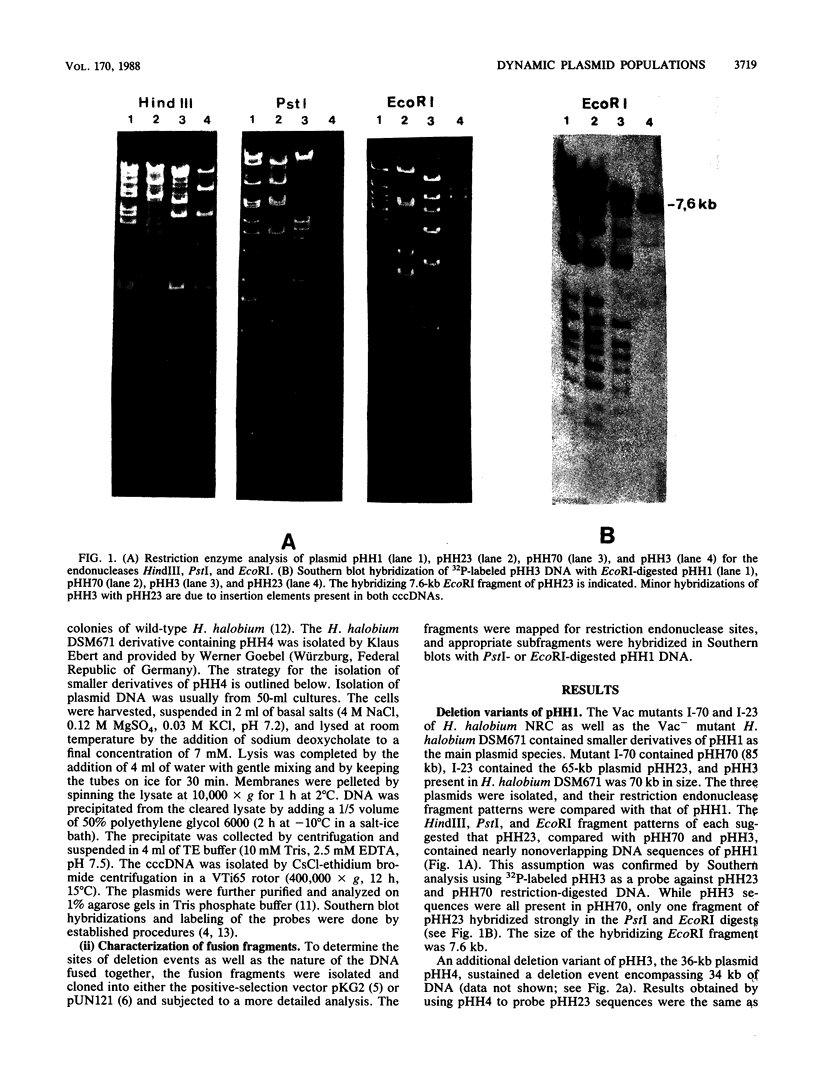
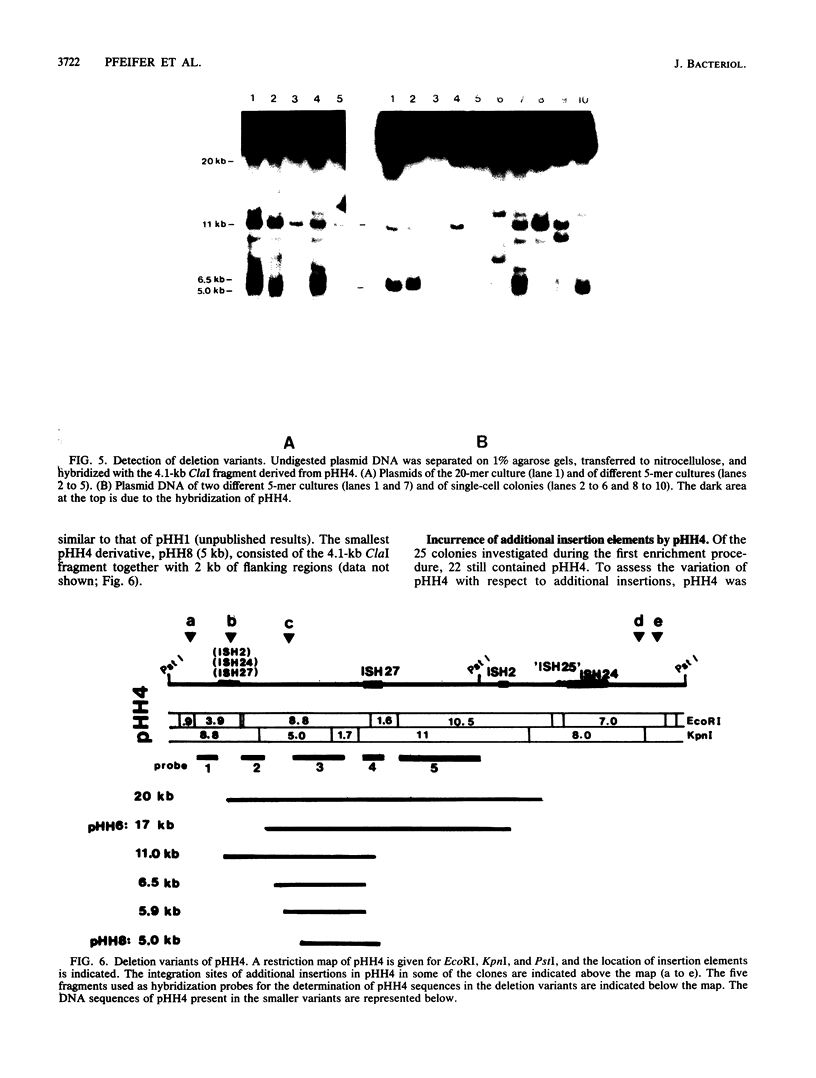
Deletion events occurring in the major 150-kilobase-pair (kb) plasmid pHH1 of the archaebacterium Halobacterium halobium were investigated. We found four deletion derivatives of pHH1 in gas-vacuole-negative mutants, two of which (pHH23) [65 kb] and pHH4 [36 kb]) we analyzed. Both plasmids incurred more than one deletion, leading to the fusion of noncontiguous pHH1 sequences. pHH23 and pHH4 overlapped by only 4 kb of DNA sequence. A DNA fragment derived from this region was used to monitor the production of further deletion variants of pHH4. A total of 25 single colonies were characterized, 23 of which contained various smaller pHH4 derivatives. Of the 25 colonies investigated, 2 had lost pHH4 entirely and contained only large (greater than or equal to 100-kb) minor covalently closed circular DNAs. One colony contained the 17-kb deletion derivative pHH6 without any residual pHH4. The sizes of the pHH4 deletion derivatives, produced during the development of a single colony, ranged from 5 to 20 kb. In five colonies, pHH4 was altered by the integration of an additional insertion element. These insertions, as well as copies of the various insertion elements already present in pHH4, presumably serve as hot spots for recombination events which result in deletions. A second enrichment procedure led to the identification of colonies containing either a 16-kb (pHH7) or a 5-kb (pHH8) deletion derivative of pHH4 as the major plasmid. pHH8, the smallest plasmid found, contained the 4 kb of unique DNA sequence shared by pHH23 and pHH4, as well as some flanking pHH4 sequences. This result indicates that the 4-kb region contains the necessary sequences for plasmid maintenance and replication.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- DasSarma S., RajBhandary U. L., Khorana H. G. High-frequency spontaneous mutation in the bacterio-opsin gene in Halobacterium halobium is mediated by transposable elements. Proc Natl Acad Sci U S A. 1983 Apr;80(8):2201–2205. doi: 10.1073/pnas.80.8.2201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Kuhn I., Stephenson F. H., Boyer H. W., Greene P. J. Positive-selection vectors utilizing lethality of the EcoRI endonuclease. Gene. 1986;42(3):253–263. doi: 10.1016/0378-1119(86)90229-5. [DOI] [PubMed] [Google Scholar]
- Pfeifer F. A., Boyer H. W., Betlach M. C. Restoration of bacterioopsin gene expression in a revertant of Halobacterium halobium. J Bacteriol. 1985 Oct;164(1):414–420. doi: 10.1128/jb.164.1.414-420.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeifer F., Betlach M. Genome organization in Halobacterium halobium: a 70 kb island of more (AT) rich DNA in the chromosome. Mol Gen Genet. 1985;198(3):449–455. doi: 10.1007/BF00332938. [DOI] [PubMed] [Google Scholar]
- Pfeifer F., Friedman J., Boyer H. W., Betlach M. Characterization of insertions affecting the expression of the bacterio-opsin gene in Halobacterium halobium. Nucleic Acids Res. 1984 Mar 12;12(5):2489–2497. doi: 10.1093/nar/12.5.2489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeifer F., Weidinger G., Goebel W. Genetic variability in Halobacterium halobium. J Bacteriol. 1981 Jan;145(1):375–381. doi: 10.1128/jb.145.1.375-381.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Sapienza C., Rose M. R., Doolittle W. F. High-frequency genomic rearrangements involving archaebacterial repeat sequence elements. Nature. 1982 Sep 9;299(5879):182–185. doi: 10.1038/299182a0. [DOI] [PubMed] [Google Scholar]
- Schnabel H. An immune strain of Halobacterium halobium carries the invertible L segment of phage PhiH as a plasmid. Proc Natl Acad Sci U S A. 1984 Feb;81(4):1017–1020. doi: 10.1073/pnas.81.4.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simsek M., DasSarma S., RajBhandary U. L., Khorana H. G. A transposable element from Halobacterium halobium which inactivates the bacteriorhodopsin gene. Proc Natl Acad Sci U S A. 1982 Dec;79(23):7268–7272. doi: 10.1073/pnas.79.23.7268. [DOI] [PMC free article] [PubMed] [Google Scholar]