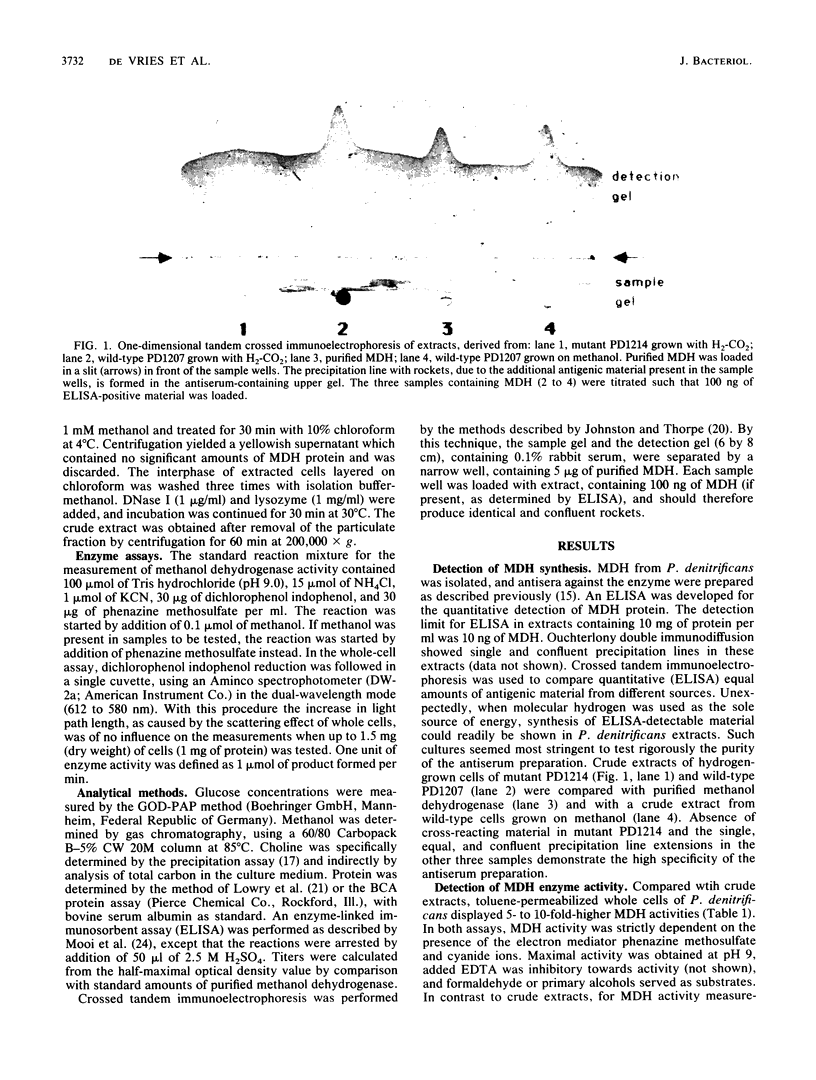
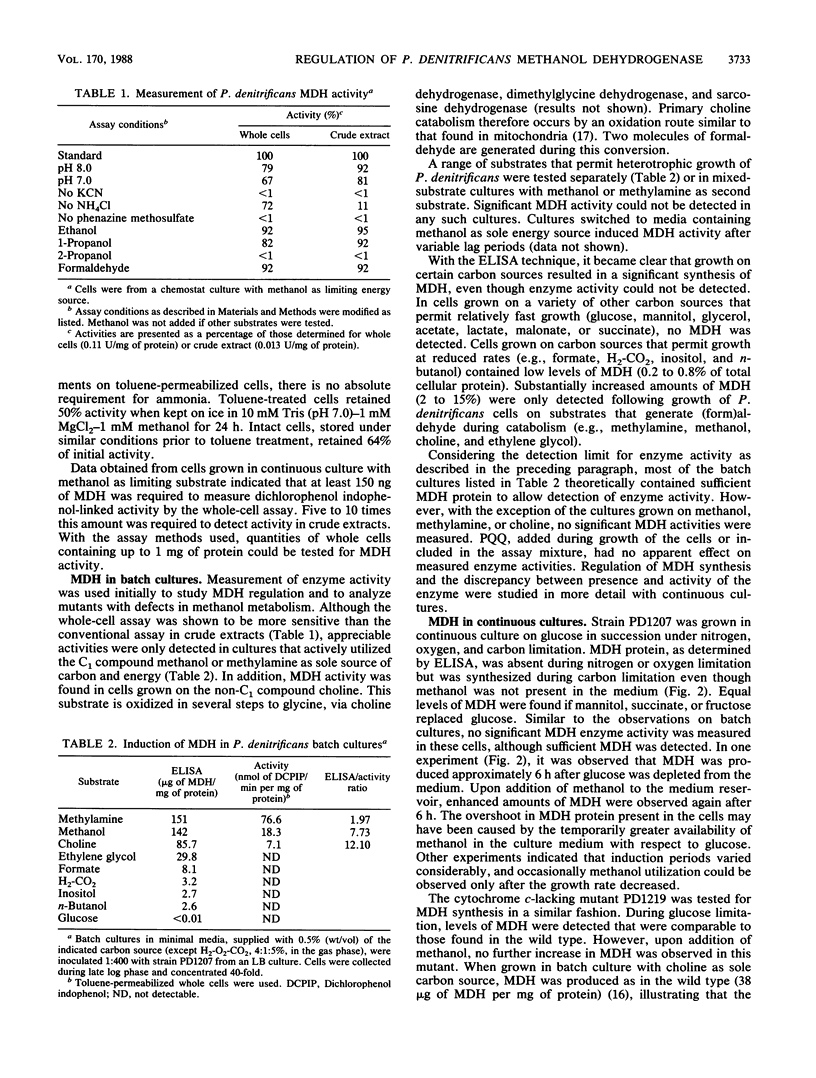
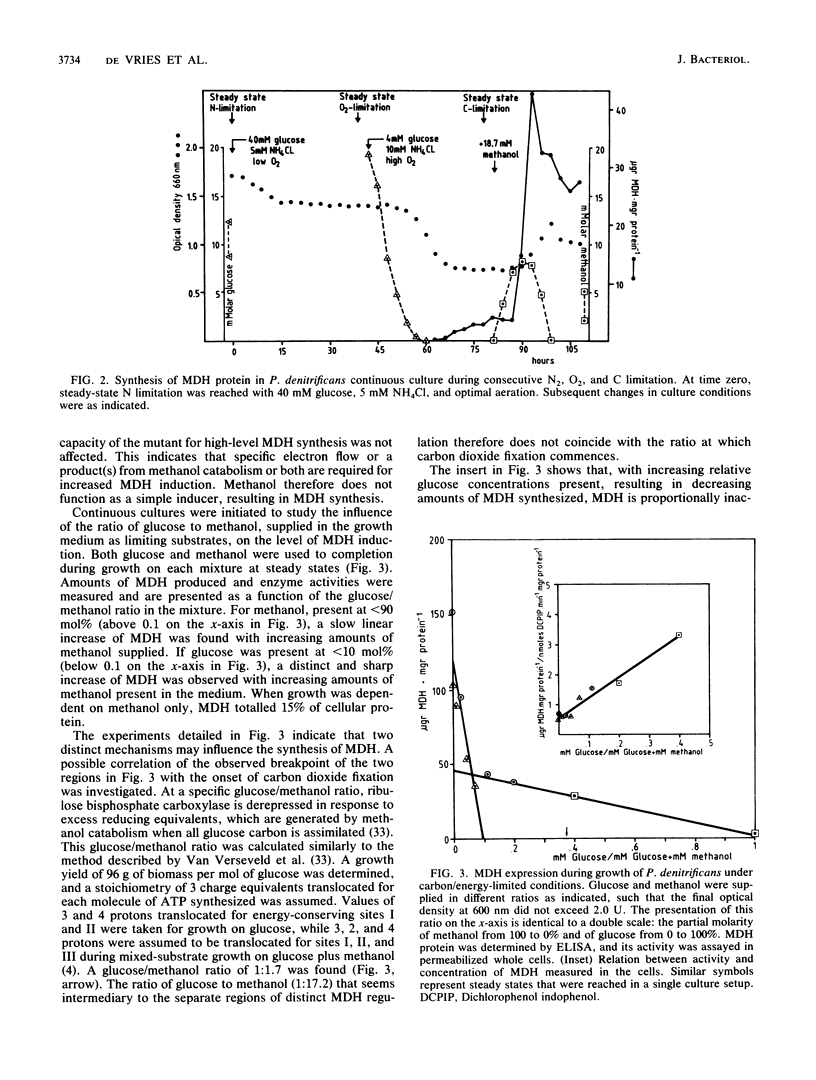
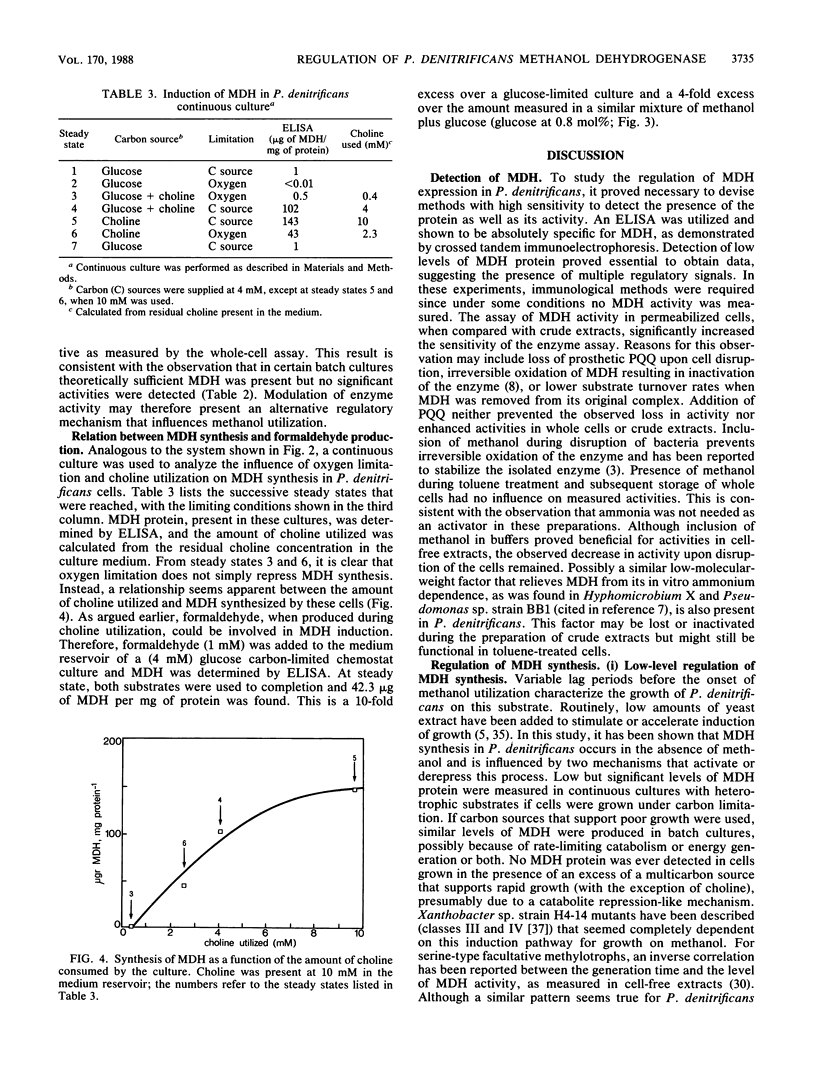
Abstract
An enzyme-linked immunosorbent assay and a whole-cell activity assay were developed which allowed detection of methanol dehydrogenase (MDH) of Paracoccus denitrificans with increased sensitivity. By these methods, it was shown that MDH was not induced by its natural substrate, methanol. Relief from a catabolite repression-like mechanism seemed responsible for low-level MDH synthesis, while product induction was the hypothesized mechanism for synthesis of high amounts of MDH. In the latter process, formaldehyde may play an important role as effector. For a variety of culture conditions, inconsistencies were observed in the relation between amounts of MDH protein synthesized and enzyme activities measured in vitro. Regulation of pyrrolo-quinoline-quinone biosynthesis or a modulation of its incorporation and stability in MDH may constitute an overriding mechanism to ensure a correct tuning between metabolic rates of methanol consumption and the required methanol oxidation rates.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alefounder P. R., Ferguson S. J. A periplasmic location for methanol dehydrogenase from Paracoccus denitrificans: implications for proton pumping by cytochrome aa3. Biochem Biophys Res Commun. 1981 Feb 12;98(3):778–784. doi: 10.1016/0006-291x(81)91179-7. [DOI] [PubMed] [Google Scholar]
- Bamforth C. W., Quayle J. R. Aerobic and anaerobic growth of Paracoccus denitrificans on methanol. Arch Microbiol. 1978 Oct 4;119(1):91–97. doi: 10.1007/BF00407934. [DOI] [PubMed] [Google Scholar]
- Cox R. B., Quayle J. R. The autotrophic growth of Micrococcus denitrificans on Methanol. Biochem J. 1975 Sep;150(3):569–571. doi: 10.1042/bj1500569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunstan P. M., Anthony C., Drabble W. T. Microbial metabolism of C 1 and C 2 compounds. The role of glyoxylate, glycollate and acetate in the growth of Pseudomonas AM1 on ethanol and on C 1 compounds. Biochem J. 1972 Jun;128(1):107–115. doi: 10.1042/bj1280107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedrich C. G. Depression of hydrogenase during limitation of electron donors and derepression of ribulosebisphosphate carboxylase during carbon limitation of Alcaligenes eutrophus. J Bacteriol. 1982 Jan;149(1):203–210. doi: 10.1128/jb.149.1.203-210.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harder W., Dijkhuizen L. Physiological responses to nutrient limitation. Annu Rev Microbiol. 1983;37:1–23. doi: 10.1146/annurev.mi.37.100183.000245. [DOI] [PubMed] [Google Scholar]
- Harms N., de Vries G. E., Maurer K., Hoogendijk J., Stouthamer A. H. Isolation and nucleotide sequence of the methanol dehydrogenase structural gene from Paracoccus denitrificans. J Bacteriol. 1987 Sep;169(9):3969–3975. doi: 10.1128/jb.169.9.3969-3975.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harms N., de Vries G. E., Maurer K., Veltkamp E., Stouthamer A. H. Isolation and characterization of Paracoccus denitrificans mutants with defects in the metabolism of one-carbon compounds. J Bacteriol. 1985 Dec;164(3):1064–1070. doi: 10.1128/jb.164.3.1064-1070.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi S. I., Lin E. C. Product induction of glycerol kinase in Escherichia coli. J Mol Biol. 1965 Dec;14(2):515–521. doi: 10.1016/s0022-2836(65)80200-5. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- McNerney T., O'connor M. L. Regulation of enzymes associated with C-1 metabolism in three facultative methylotrophs. Appl Environ Microbiol. 1980 Aug;40(2):370–375. doi: 10.1128/aem.40.2.370-375.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooi F. R., de Graaf F. K., van Embden J. D. Cloning, mapping and expression of the genetic determinant that encodes for the K88ab antigen. Nucleic Acids Res. 1979 Mar;6(3):849–865. doi: 10.1093/nar/6.3.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Netrusov A. I., Anthony C. The microbial metabolism of C1 compounds. Oxidative phosphorylation in membrane preparations of Pseudomonas AM1. Biochem J. 1979 Feb 15;178(2):353–360. doi: 10.1042/bj1780353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunn D. N., Lidstrom M. E. Isolation and complementation analysis of 10 methanol oxidation mutant classes and identification of the methanol dehydrogenase structural gene of Methylobacterium sp. strain AM1. J Bacteriol. 1986 May;166(2):581–590. doi: 10.1128/jb.166.2.581-590.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunn D. N., Lidstrom M. E. Phenotypic characterization of 10 methanol oxidation mutant classes in Methylobacterium sp. strain AM1. J Bacteriol. 1986 May;166(2):591–597. doi: 10.1128/jb.166.2.591-597.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanier R. Y., Ornston L. N. The beta-ketoadipate pathway. Adv Microb Physiol. 1973;9(0):89–151. [PubMed] [Google Scholar]
- Van Verseveld H. W., Stouthamer A. H. Electron-transport chain and coupled oxidative phosphorylation in methanol-grown Paracoccus denitrificans. Arch Microbiol. 1978 Jul;118(1):13–20. doi: 10.1007/BF00406068. [DOI] [PubMed] [Google Scholar]
- Weaver C. A., Lidstrom M. E. Isolation, complementation and partial characterization of mutants of the methanol autotroph Xanthobacter H4-14 defective in methanol dissimilation. J Gen Microbiol. 1987 Jul;133(7):1721–1731. doi: 10.1099/00221287-133-7-1721. [DOI] [PubMed] [Google Scholar]
- Weaver C. A., Lidstrom M. E. Methanol dissimilation in Xanthobacter H4-14: activities, induction and comparison to Pseudomonas AM1 and Paracoccus denitrificans. J Gen Microbiol. 1985 Sep;131(9):2183–2197. doi: 10.1099/00221287-131-9-2183. [DOI] [PubMed] [Google Scholar]
- van Verseveld H. W., Krab K., Stouthamer A. H. Proton pump coupled to cytochrome c oxidase in Paracoccus denitrificans. Biochim Biophys Acta. 1981 May 13;635(3):525–534. doi: 10.1016/0005-2728(81)90111-0. [DOI] [PubMed] [Google Scholar]



