Abstract
Sulfation is critical to the function of a wide variety of biomolecules. This common modification requires the enzymatic synthesis of an activated sulfate donor, phosphoadenosine-phosphosulfate (PAPS). In higher organisms PAPS synthesis is catalyzed by a bifunctional sulfurylase kinase (SK) polypeptide having both ATP-sulfurylase and adenosine-phosphosulfate kinase activities. We report the identification of a gene family encoding murine SK proteins with these two activities. A family member, SK2, colocalizes with the locus for the autosomal recessive murine phenotype brachymorphism. Brachymorphic mice have normal lifespans, but abnormal hepatic detoxification, bleeding times, and postnatal growth, the latter being attributed to undersulfation of cartilage proteoglycan. A missense mutation in the SK2 coding sequence of bm mice that alters a highly conserved amino acid residue destroys adenosine-phosphosulfate kinase activity and therefore the ability of SK2 to synthesize PAPS. We conclude that a family of SK genes are responsible for sulfate activation in mammals, that a mutation in SK2 causes murine brachymorphism, and that members of this gene family have nonredundant, tissue-specific roles.
Sulfation is a necessary modification of proteins, carbohydrates, and lipids. One of the most prominent roles of sulfation in vertebrates is the posttranslational modification of glycosaminoglycans, the repeating disaccharide chains that are covalently linked to protein cores to constitute the family of proteoglycans. Sulfation requires active transport of sulfate into the cell, conversion into the “active” high energy form of phosphoadenosine-phosphosulfate (PAPS), translocation of PAPS across the golgi membrane, and transfer of sulfate from PAPS, via a multitude of sulfotransferases, to the recipient biomolecules (1). The sulfate activation pathway consists of two activities, ATP-sulfurylase (EC 2.7.7.4), which catalyzes synthesis of adenosine-phosphosulfate (APS) from ATP and SO4−2, and APS kinase (EC 2.7.1.25), which phosphorylates APS in the presence of another molecule of ATP to form PAPS. In simpler organisms these activities are catalyzed by separate enzymes (2–5). In contrast, when these two sulfate-activating activities were purified from rat chondrosarcoma they were found to exist as a bifunctional enzyme (6, 7), which uses a channeling mechanism to transfer the intermediate APS efficiently from the sulfurylase to the kinase active site (8–10). Subsequently, a mouse cDNA encoding a fused ATP-sulfurylase/APS kinase was isolated. This mammalian PAPS synthetase (referred to as SK1) is a bifunctional polypeptide that catalyzes both ATP-sulfurylase and APS-kinase reactions (11).
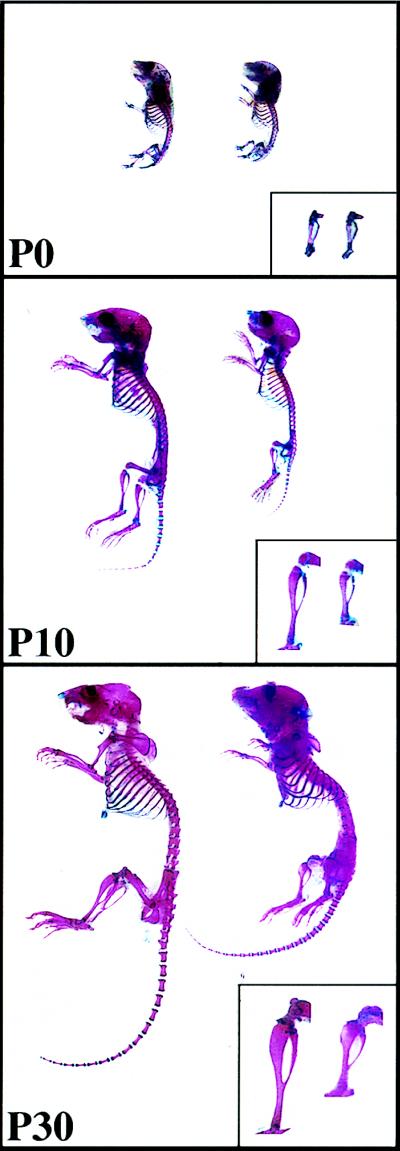
Murine brachymorphism is characterized by dome-shaped skull, short thick tail, and shortened but not widened limbs (12, 13) and has been attributed to a defective PAPS synthesis pathway (14–17). The phenotype is inherited as an autosomal recessive, and bm homozygotes breed normally, have life spans comparable to wild-type mice, and are about the same size as normals at birth. The difference in size becomes apparent over the first 4 weeks of life, resulting in a 50% reduction in limb length and a 25% reduction in axial skeletal size (Fig. 1). The effect on growth is concomitant with a progressive reduction in size of the columnar and hypertrophic zones in the epiphyseal growth plates. Histological and ultrastructual studies suggest a defective cartilage matrix that contains normal collagen fibrils, but proteoglycan aggregate granules that are smaller than normal and present in reduced numbers, particularly in the growth plate (18). Biochemical analysis showed that brachymorphic cartilage contains normal levels of glycosaminoglycans but with disaccharides that are significantly undersulfated. The reduced incorporation of sulfate in brachymorphic cartilage is associated with limited PAPS availability because of a reduction predominantly in APS-kinase activity (14–17). Genetic linkage studies localized the bm gene on chromosome 19 (12, 19). However, the precise gene responsible for brachymorphism has not been identified.
Figure 1.
Postnatal skeletal development of normal (Left) and bm (Right) mice. Calcified bone and cartilage were differentially stained with Alzarin red and Alcian blue, respectively, at zero (P0), 10 (P10), and 30 (P30) days after birth. (Insets) Tibias of normal (Left) and bm (Right) mice. Note the similar size in all of the skeletal elements at P0 and the slower increases in the length of the brachymorphic skeletal elements.
Here we report the isolation of an additional member of the PAPS synthetase family, SK2, and the chromosomal localization of both SK1 and SK2 genes. SK2 is mapped on chromosome 19 and is tightly linked with the marker for bm locus (19). Sequence analysis of a bm SK2 cDNA revealed a missense mutation that results in a glycine-to-arginine substitution at a highly conserved portion of the APS-kinase domain, and bacterially expressed bm SK2 fails to catalyze the APS-kinase reaction and to synthesize PAPS. These findings indicate that the mutation in the SK2 gene is responsible for murine brachymorphism.
MATERIALS AND METHODS
Identification and Cloning of SK2.
The deduced amino acid sequence of SK1 (GenBank accession no. U34883) was used as a blast query sequence against the expressed sequence tag (EST) database maintained by the National Center for Biotechnology Information at the National Library of Medicine. Nucleotide sequences from several EST clones suspected to contain SK2 cDNA initially were used to generate oligonucleotide primers. The 5′-end of a cDNA sequence subsequently was obtained by the 5′-inverse PCR method (20). Reverse transcription–PCR (RT-PCR) was performed on total RNA extracted from normal and bm neonatal livers. The first strand was synthesized by SuperScript II (GIBCO/BRL) using an antisense primer (SK5, 5′-GCAATTGGATACAGAGCAGC-3′) complementary to the 3′-untranslated region of SK2 mRNA. The complete ORF cDNA was amplified with a 5′-end sense primer containing a NdeI site (SK30, 5′-AGAGAGTTCCATATGTCTGCAAATTCCAAAATGAACCATAAAAGAGACCAGC-3′) and a 3′-end antisense primer containing a XhoI site (SK31, 5′-GAGAGAGATCTCGAGCTAGTTGGTCTTCTCCAGAGACCTGTAGTAATCTGTCAACAC-3′) using Expand (Boehringer-Mannheim). RT-PCR of the total liver RNA yielded a single band approximately 1.9 kb in length. The fragment was digested with NdeI and XhoI and cloned into appropriate restriction sites in a bacterial expression vector, pET-15b (Novagen). The nucleotide sequence was determined by the dideoxynucleotide chain termination method using T7 sequenase kit (Amersham). To confirm the bm SK2 mutation at nucleotide 235, genomic DNA sequence at the nucleotide 235 site from the normal and bm mice was determined after amplifying genomic DNA fragments by PCR using a set of two primers flanking the mutation site (SK15, 5′-CAGTAGATCTCGAGTGCGGATATTCTCTTCTCGGTCC-3′; SK26, 5′-CAACAATAAGCTTTCCTTTGGAAGAGTACC-3′).
Northern Blot Analysis.
Livers were dissected from neonatal normal and bm mice and immediately frozen on dry ice, and total RNA was isolated by using TRIzol (GIBCO/BRL). Fifteen micrograms of total liver RNA was electrophoresed in a 1% agarose-formaldehyde gel and transferred to a Nytran filter. Prehybridization, hybridization with the 32P-labeled 1.9-kb SK2 cDNA, and washing were performed as previously described (11). The same filter was stripped and rehybridized with a mouse β-actin cDNA probe.
Recombinant Enzyme Assays.
An Escherichia coli strain, BL21(DE3), was transformed by the heat-shock method with a SK2 cDNA cloned in pET-15b vector (Novagen). Isopropyl β-d-thiogalactoside (10 mM) was added to an overnight bacterial culture and incubated for an additional 4 hr. The bacterial cell cultures were centrifuged at 9,000 × g, and the pellet was resuspended into IMAC 5 buffer (5 mM imidazole/50 mM Tris, pH 7.9). The protein was purified after cell sonication and gravity His-tag purification (Novagen) and quantitated by the Pierce BCA protein assay method. Purified enzyme was dialysed into phosphate buffer (25 mM NaH2PO4-K2HPO4, pH 7.8/1 mM DTT/1 mM EDTA) overnight and diluted with phosphate buffer to 20 μg/ml for enzymatic assays. Crude extracts from bacteria carrying the vector without insert and no vector were used as negative controls. The ATP-sulfurylase was assayed in the reverse direction of ATP formation as described (8). Standard ATP-sulfurylase assays contained 50 mM NaH2PO4-K2HPO4 (pH 7.8), 12 mM MgCl2, 0.5 mM DTT, 5 mM NaF, 0.2 mM Na4P2O7 (containing 6.7 mCi of 32P), 0.1 mM APS, and 50 μl of enzyme preparation. APS kinase was assayed as described (10). Standard APS-kinase assay contained 80 nM [35S]APS, 0.5 mM ATP (pH 7.0), 5 mM MgCl2, 10 mM ammonium sulfate, and 12 μl of enzyme, brought up to 25 μl with buffer A (25 mM NaH2PO4-K2HPO4, pH 7.8/1 mM DTT/1 mM EDTA/10% glycerol). The overall reaction with ATP and SO42− as substrates and measuring the production of APS and PAPS was assayed as described (6). The 25-μl standard overall reaction contained 0.4 mM [35S]H2SO4, 10 mM ATP, 20 mM MgCl2, 22 mM Tris⋅HCl (pH 8.0), and 15 μl of enzyme preparation.
Chromosomal Localization.
A murine SK1 PCR product from primer pair SK16 (5′-GGAAACGGCCTGATTTCAGG-3′) and SK21 (5′-CACGCGAAGGCCAGAAACC-3′) was used to amplify a 190-bp intragenic SK1 fragment from C57BL/6J and Mus spretus genomic DNA. The resulting amplimers were separated on a nondenaturing 0.5× mutation detection enhancement (MDE) (FMC) gel at 6 W for 20 hr. Amplimers were visualized by autoradiography because the SK16 primer had been end-labeled with 32P before PCR amplification. A single-strand conformational polymorphism (SSCP) (21) was detected between C57BL/6J and M. spretus; this SSCP then was evaluated on the BSS backcross mapping panel available from the Jackson Laboratory (22). Offspring were scored as homozygous for the spretus allele or heterozygous for C57BL/6J and spretus alleles. Results were submitted to the Jackson Laboratory, where precise mapping was determined relative to previously tested markers.
Murine SK2 was mapped by using the same approach, but with the murine SK2-specific primer pair SK12 (5′-GGAGAAAACCAACTAGGTGCTCCTGGC-3′) and SK13 (5′-CATAGTGGCTGAGATGAAGTGTACAGTGC-3′). This pair amplifies a 287-bp intragenic fragment of the SK2 gene, which also has a single-strand conformational polymorphism between C57BL/6J and M. spretus. Offspring of the BSS backcross were scored for this SK2 polymorphism and results were submitted to the Jackson Laboratory. SK2 was found to cosegregate with D19Mit13 (logarithm of odds 28.3 at θ = 0) The human SK genes were placed on the genetic map via radiation hybrid mapping (23).
RESULTS
Cloning and Characterization of a SK2 cDNA.
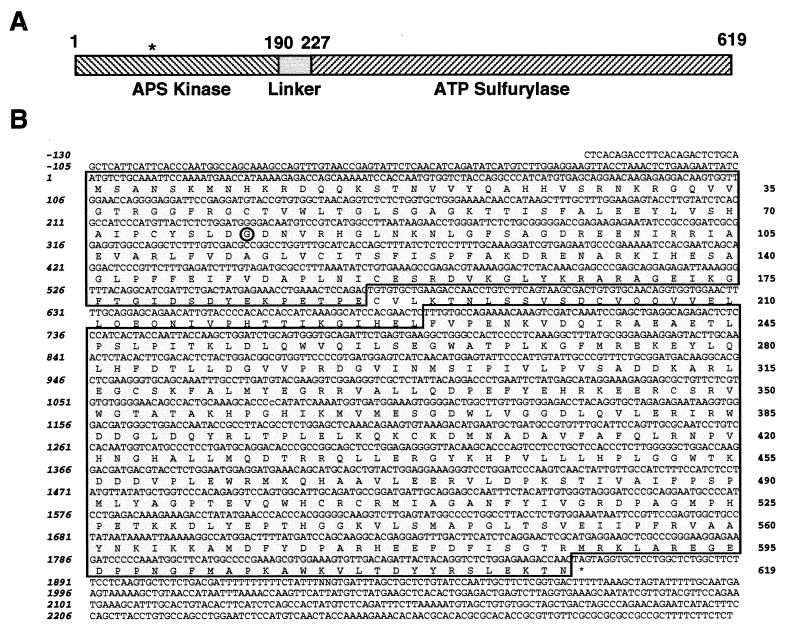
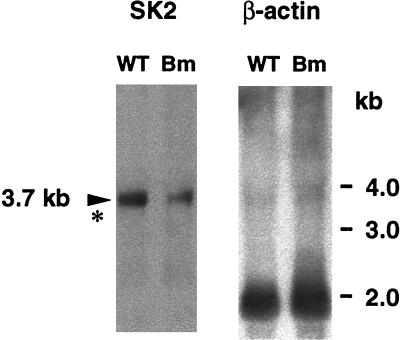
The earlier cloning of the bifunctional murine SK (SK1) (11) did not identify the underlying etiology of the brachymorphic phenotype. Furthermore, we genetically mapped SK1 to mouse chromosome 3, which is also incompatible with it being the cause of brachymorphism, which maps to chromosome 19 (12). These results led us to consider the possibility that more than one SK gene might be present in mice. A second SK isoform first was detected after sequence similarity searching (24, 25) using murine SK1 as the query sequence. Two groups of murine ESTs were identified; one group having near identity to the query sequence and presumably representing SK1 transcripts. The other group of ESTs had lower nucleotide homology, but high amino acid identity, and potentially represents novel SK isoforms. One of these latter ESTs was used to isolate the complete cDNA of an additional isoform, referred to as SK2, from mouse total liver RNA by 5′-inverse PCR (20) (Fig. 2). SK2 contains an uninterrupted coding sequence of 1,863 bp and has 76% identity at the amino acid level to SK1. SK2 also contains functional motifs previously associated with unifunctional ATP sulfurylases and APS kinases (11). Northern blot analysis reveals a single SK2 transcript of 3.7 kb in normal and brachymorphic liver (Fig. 3), slightly larger than the 3.3-kb SK1 transcript (11). The 3.7-kb SK2 transcript also was detected in cartilage, skin, and brain (data not shown).
Figure 2.
Sequence of SK2. (A) Schematic diagram of ATP sulfurylase/APS kinase. Approximate location of the mutation in bm SK2 is indicated by ∗. (B) The cDNA sequence and its deduced amino acid sequence are shown. The mutation found in bm SK2 is circled.
Figure 3.
Northern blot analysis of SK2. Fifteen micrograms of total RNA extracted from normal and brachymorphic liver was electrophoresed in a 1% agarose-formaldehyde gel and transferred to a Nytran filter. Hybridization with a 32P-labeled 1.9-kb SK2 cDNA yielded a single band at approximately 3.7 kb. Approximate location for the 3.3-kb SK1 message is indicated by ∗. Hybridization with a mouse β-actin probe yielded a single band at approximately 1.8 kb.
Chromosomal Localization of SK1 and SK2 Genes.
SK1 was found to cosegregate with D3Xrf10, a locus on the distal end of chromosome 3 (logarithm of odds 28.3 at θ = 0), whereas SK2 was mapped to chromosome 19 by using an intragenic single-strand conformational polymorphism on an interspecific backcross mapping panel. SK2 is tightly linked to D19Mit13, as is the bm locus (19). Consequently, SK2 was tested as a candidate for causing brachymorphism.
Mutation Analysis and Recombinant SK2 Enzyme Assays.
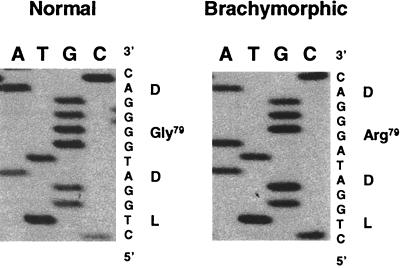
Sequence analysis of wild-type and bm SK2 cDNA revealed a single nucleotide change, a G to A transition, in the bm allele that changes a glycine residue to an arginine residue (Gly79 to Arg) in the APS-kinase domain of the bifunctional enzyme (Fig. 2). This change also is present in genomic DNA (Fig. 4). That the change causes disease is suggested by the conservation of this glycine residue in all previously characterized bifunctional SK proteins and in all unifunctional APS kinases (11). Further support for this mutation being the cause of the brachymorphic phenotype derives from in vitro assays of wild-type and mutant recombinant enzyme activities. Bacterially expressed recombinant SK2 protein was found to catalyze both ATP-sulfurylase and APS-kinase reactions, synthesizing PAPS comparably to SK1 (11). In contrast to recombinant expressed wild-type SK2, recombinant expressed SK2 protein containing the Gly79 to Arg mutation has no APS-kinase activity, although its ATP-sulfurylase activity is maintained (Fig. 5). Taken together these results indicate that this mutation is responsible for murine brachymorphism.
Figure 4.
Genomic DNA sequence at the mutation site from normal and bm mice. Position at nucleotide 235 is changed from G235 to A235 resulting in the amino acid change from Gly79 to Arg79 in the N-terminal APS kinase portion of the bm SK2.
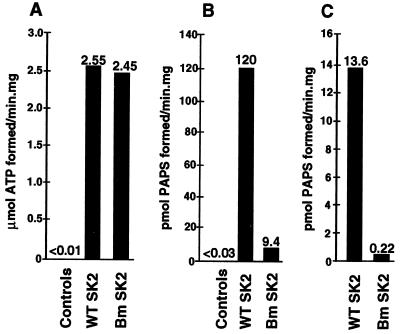
Figure 5.
Activities of the wild-type and brachymorphic SK2 enzyme. (A) ATP sulfurylase activity as measured by the reverse sulfurylase assay where formation of ATP is monitored. (B) APS kinase activity is measured as the amount of PAPS generated from APS and ATP. (C) PAPS synthetase activity is measured as the amount of PAPS generated starting from free sulfate and ATP. Controls in A and B represent DE3 cells and DE3 cells with pET-15b vector.
DISCUSSION
The activation of sulfate to produce the universal sulfate donor, PAPS, is critical to many processes, including the activation of numerous peptide and steroid hormones, the metabolism of catecholamines, the production of ceramide during brain development, the synthesis of sulfolipids in the reproductive system, the proper functioning of the blood coagulation cascade, the detoxification of endogenous compounds, and the modification of a variety of drugs, mutagens, and bile acids. Our results indicate a gene family is responsible for encoding this critical function and suggest nonredundant, tissue-specific role of each isoform, as demonstrated by the tissue-specific defect in PAPS synthesis in bm mice. Although the key steps in sulfating biomolecules have been assumed to be at the level of the myriad of sulfotransferases, our findings further demonstrate that regulation at the level of forming the sulfate donor is important as well, suggesting possible regulatory mechanisms at other steps along the overall sulfation pathway. Finally, the finding of additional ESTs (unpublished data), which have reduced nucleotide identity but high amino acid identity to SK1 and SK2, suggests that this family will likely grow.
It is intriguing that the SK2 mutation specifically affects postnatal skeletal development and other selected tissues. Newborn bm mice appear skeletally normal, suggesting that the SK2 gene product’s principal role during skeletogenesis is during postnatal growth and that other SKs may substitute for SK2 in utero. It has been shown that hepatic detoxification in bm mice is principally via glucuronidation, whereas it is via sulfation in wild-type mice (26). Bleeding times are also abnormal in bm mice (19). However, other tissues that contain important sulfated biomolecules such as the kidney and brain appear normal in bm mice, suggesting tissue-specific sulfation mechanisms (17). Although the SK2 transcript is found in total brain RNA, bm mice do not have an obvious neurologic phenotype. Careful neurological testing of bm homozygotes now is indicated to determine whether there may be subtle neurological effects, although absence of such a phenotype simply may represent functional redundancy for sulfate activation in the brain or a minor role for SK2 in this tissue. The contribution of each SK isoform during growth and development must await the identification of all family members and careful quantitation of their mRNA and protein levels. However, because it is now clear that the PAPS synthetases constitute a gene family, with known members lacking complete functional redundancy at the tissue level and with perhaps more members yet to be discovered, the bm mouse is an excellent model to study the role of sulfation in a variety of specific processes.
There are not yet human counterparts for the bm or other SK mutant phenotypes. Radiation hybrid mapping of the human homologues of SK1 and SK2 place them on chromosomes 4q and 10q, respectively (unpublished data), consistent with the locations of other human genes whose mouse homologues are syntenic with the SK loci. At present no likely murine phenotype colocalizes with the SK1 locus, nor have likely human phenotypes been mapped to regions containing the human SK homologues. However, human genetic disorders have been associated with mutations in other components of the sulfate metabolic pathway; for example, mutations at the locus encoding the high affinity sulfate transporter (DTDST), responsible for uptake of inorganic sulfate (one of the two substrates for SKs) into the cell, are associated with skeletal phenotypes also having undersulfated proteoglycans (27–29). Consequently, one would predict that mutations in SK1 and SK2 also will have consequences in humans.
Acknowledgments
We thank Lucy Rowe and Mary Barter for assistance in placing the SK1 and SK2 loci into the murine genetic map, Mary Lou Spach for maintenance of the brachymorphic colony, Judith Henry for tissue preparations, Glenn Burrell for manuscript preparation, and James Mensch for manuscript review. This work was supported by grants from the National Institute of Child Health and Human Development, the National Institute of Arthritis and Musculoskeletal and Skin Diseases, the Mitzutani Foundation, and the March of Dimes Foundation.
ABBREVIATIONS
- SK
sulfurylase kinase
- PAPS
phosphoadenosine-phosphosulfate
- APS
adenosine-phosphosulfate
- EST
expressed sequence tag
Footnotes
Data deposition: The SK2 cDNA sequence reported in this paper has been deposited in the GenBank database (accession no. AF052453).
References
- 1.Schwartz N B, Lyle S, Ozeran J D, Li H, Deyrup A, Ng K, Westley J. Chem Biol Interact. 1998;109:143–151. doi: 10.1016/s0009-2797(97)00129-4. [DOI] [PubMed] [Google Scholar]
- 2.Korch C, Mountain H A, Bystrom A S. Mol Gen Genet. 1991;229:96–108. doi: 10.1007/BF00264218. [DOI] [PubMed] [Google Scholar]
- 3.Leyh T S, Vogt T F, Suo Y. J Biol Chem. 1992;267:10405–10410. [PubMed] [Google Scholar]
- 4.Jain A, Leustek T. Plant Physiol. 1994;105:771–772. doi: 10.1104/pp.105.2.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schwedock J S, Liu C, Leyh T S, Long S R. J Bacteriol. 1994;176:7055–7064. doi: 10.1128/jb.176.22.7055-7064.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lyle S, Stanzak J, Ng K, Schwartz N B. Biochemistry. 1994;33:5920–5925. doi: 10.1021/bi00185a032. [DOI] [PubMed] [Google Scholar]
- 7.Geller D, Henry J G, Belch J, Schwartz N B. J Biol Chem. 1987;262:7374–7382. [PubMed] [Google Scholar]
- 8.Lyle S, Geller D H, Ng K, Stanzak J, Westley J, Schwartz N B. Biochem J. 1994;301:355–359. doi: 10.1042/bj3010355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lyle S, Ozeran J D, Stanzak J, Westley J, Schwartz N B. Biochemistry. 1994;33:6822–6827. doi: 10.1021/bi00188a010. [DOI] [PubMed] [Google Scholar]
- 10.Lyle S, Stanzak J, Westley J, Schwartz N B. Biochemistry. 1995;34:940–945. doi: 10.1021/bi00003a028. [DOI] [PubMed] [Google Scholar]
- 11.Li H, Deyrup A, Mensch J, Domowicz M, Konstantinidis A, Schwartz N B. J Biol Chem. 1995;270:29453–29459. doi: 10.1074/jbc.270.49.29453. [DOI] [PubMed] [Google Scholar]
- 12.Lane P W, Dickie M M. J Hered. 1968;65:297–300. doi: 10.1093/oxfordjournals.jhered.a107725. [DOI] [PubMed] [Google Scholar]
- 13.Schwartz, N. B. & Domowicz, M. (1998) Am. Assoc. Orthoped. Surg. Publ., in press.
- 14.Schwartz N B, Ostrowski V, Brown K S, Pratt R. Biochem Biophys Res Commun. 1978;82:173–178. doi: 10.1016/0006-291x(78)90592-2. [DOI] [PubMed] [Google Scholar]
- 15.Sugahara K, Schwartz N B. Proc Natl Acad Sci USA. 1979;76:6615–6618. doi: 10.1073/pnas.76.12.6615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sugahara K, Schwartz N B. Arch Biochem Biophys. 1982;214:589–601. doi: 10.1016/0003-9861(82)90064-9. [DOI] [PubMed] [Google Scholar]
- 17.Sugahara K, Schwartz N B. Arch Biochem Biophys. 1982;214:601–609. doi: 10.1016/0003-9861(82)90064-9. [DOI] [PubMed] [Google Scholar]
- 18.Orkin R W, Williams B R, Cranley R E, Poppke D C, Brown K S. J Cell Biol. 1977;73:287–299. doi: 10.1083/jcb.73.2.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rusiniak M E, O’Brien E P, Novak E K, Barone S M, McGarry M P, Reddington M, Swank R T. Mamm Genome. 1996;7:98–102. doi: 10.1007/s003359900027. [DOI] [PubMed] [Google Scholar]
- 20.Zeiner M, Gehring U. BioTechniques. 1994;17:1051–1053. [PubMed] [Google Scholar]
- 21.Orita M, Iwahana H, Kanazawa H, Hayashi K, Sekiya T. Proc Natl Acad Sci USA. 1989;86:2766–2770. doi: 10.1073/pnas.86.8.2766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rowe L B, Nadeau J H, Turner R, Frankel W N, Letts V A, Eppig J T, Ko M S H, Thurston S J, Birkenmeier E H. Mamm Genome. 1994;5:253–274. doi: 10.1007/BF00389540. [DOI] [PubMed] [Google Scholar]
- 23.Cox D R. Cytogenet Cell Genet. 1992;59:80–81. doi: 10.1159/000133205. [DOI] [PubMed] [Google Scholar]
- 24.Altschul S F, Gish W, Miller W, Myers E W, Lipman D J. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 25.Boguski M S, Lowe T M, Tolstoshev C M. Nat Genet. 1993;4:332–333. doi: 10.1038/ng0893-332. [DOI] [PubMed] [Google Scholar]
- 26.Schwartz N B. In: Limb Development and Regeneration. Kelley R O, Goetinck P F, MacCabe J A, editors. New York: Liss; 1983. , Part B, pp. 97–103. [Google Scholar]
- 27.Hastbacka J, Chapelle A d l, Mahtani M M, Clines G, Reeve-Daly M P, Hamilton B A, Kusumi K, Trivedi B, Weaver A, Coloma A, et al. Cell. 1994;78:1073–1078. doi: 10.1016/0092-8674(94)90281-x. [DOI] [PubMed] [Google Scholar]
- 28.Hastbacka J, Wilcox W R, Superti-Furga A, Rimoin D L, Cohn D H, Lander E S. Am J Hum Genet. 1996;58:255–262. [PMC free article] [PubMed] [Google Scholar]
- 29.Superti-Furga A, Hastbacka J, Wilcox W R, Cohn D H, van der Harten H J, Rossi A, Blau N, Rimoin D L, Steinmann B, Lander E S, et al. Nat Genet. 1996;12:100–102. doi: 10.1038/ng0196-100. [DOI] [PubMed] [Google Scholar]