Abstract
Saccharomyces cerevisiae ascospores germinate in the presence of acetate without any detectable trehalose degradation, as revealed by high-resolution nuclear magnetic resonance spectroscopy and by a standard colorimetric assay. The results presented here substantiate the hypothesis that in S. cerevisiae trehalose supplies energy during dormancy of the spores and not during the germination process.
Full text
PDF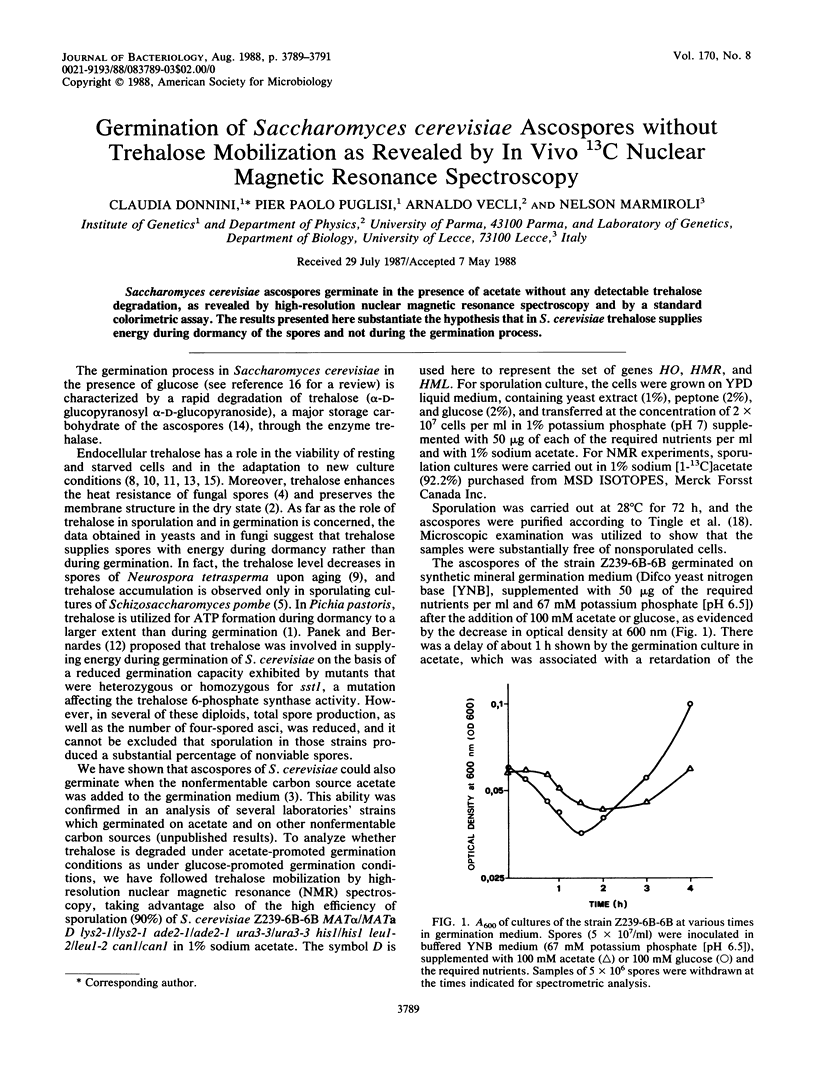

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barton J. K., Den Hollander J. A., Hopfield J. J., Shulman R. G. 13C nuclear magnetic resonance study of trehalose mobilization in yeast spores. J Bacteriol. 1982 Jul;151(1):177–185. doi: 10.1128/jb.151.1.177-185.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowe J. H., Crowe L. M., Chapman D. Preservation of membranes in anhydrobiotic organisms: the role of trehalose. Science. 1984 Feb 17;223(4637):701–703. doi: 10.1126/science.223.4637.701. [DOI] [PubMed] [Google Scholar]
- Donnini C., Artoni N., Marmiroli N. Germination conditions that require mitochondrial function in Saccharomyces cerevisiae: utilization of acetate and galactose. J Bacteriol. 1986 Dec;168(3):1250–1253. doi: 10.1128/jb.168.3.1250-1253.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emyanitoff R. G., Wright B. E. Effect of intracellular carbohydrates on heat resistance of Dictyostelium discoideum spores. J Bacteriol. 1979 Dec;140(3):1008–1012. doi: 10.1128/jb.140.3.1008-1012.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue H., Shimoda C. Induction of trehalase activity on a nitrogen-free medium: a sporulation-specific event in the fission yeast, Schizosaccharomyces pombe. Mol Gen Genet. 1981;183(1):32–36. doi: 10.1007/BF00270134. [DOI] [PubMed] [Google Scholar]
- Kane S. M., Roth R. Carbohydrate metabolism during ascospore development in yeast. J Bacteriol. 1974 Apr;118(1):8–14. doi: 10.1128/jb.118.1.8-14.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lillie S. H., Pringle J. R. Reserve carbohydrate metabolism in Saccharomyces cerevisiae: responses to nutrient limitation. J Bacteriol. 1980 Sep;143(3):1384–1394. doi: 10.1128/jb.143.3.1384-1394.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lingappa B. T., Sussman A. S. Endogenous Substrates of Dormant, Activated and Germinating Ascospores of Neurospora Tetrasperma. Plant Physiol. 1959 Jul;34(4):466–472. doi: 10.1104/pp.34.4.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rousseau P., Halvorson H. O., Bulla L. A., Jr, St Julian G. Germination and outgrowth of single spores of Saccharomyces cerevisiae viewed by scanning electron and phase-contrast microscopy. J Bacteriol. 1972 Mar;109(3):1232–1238. doi: 10.1128/jb.109.3.1232-1238.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thevelein J. M. Regulation of trehalose mobilization in fungi. Microbiol Rev. 1984 Mar;48(1):42–59. doi: 10.1128/mr.48.1.42-59.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thevelein J. M., den Hollander J. A., Shulman R. G. Changes in the activity and properties of trehalase during early germination of yeast ascospores: correlation with trehalose breakdown as studied by in vivo 13C NMR. Proc Natl Acad Sci U S A. 1982 Jun;79(11):3503–3507. doi: 10.1073/pnas.79.11.3503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tingle M. A., Küenzi M. T., Halvorson H. O. Germination of yeast spores lacking mitochondrial deoxyribonucleic acid. J Bacteriol. 1974 Jan;117(1):89–93. doi: 10.1128/jb.117.1.89-93.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]



