Abstract
In the chloroplasts of higher plants and algae, the biosynthesis of the chlorophyll precursor delta-aminolevulinic acid (ALA) involves at least three enzymes and a tRNA species. Here we demonstrate that in cell extracts of the unicellular cyanobacterium Synechocystis sp. strain PCC 6803 ALA was formed from glutamate in a series of reactions in which activation of glutamate by glutamyl-tRNAGlu formation was the first step. The activated glutamate was reduced by a dehydrogenase which displayed tRNA sequence specificity. Fractionation of strain 6803 tRNA by reverse-phase chromatography and polyacrylamide gel electrophoresis yielded two pure tRNAGlu species which stimulated ALA synthesis in vitro. These tRNAs had identical primary sequences but differed in the nucleotide modification of their anticodon. The 6803 tRNAGlu was similar to the sequences of tRNAGlu species or tRNAGlu genes from Escherichia coli and from chloroplasts of Euglena gracilis and higher plants. Southern blot analysis revealed at least two tRNAGlu gene copies in the 6803 chromosome. A glutamate-1-semialdehyde aminotransferase, the terminal enzyme in the conversion of glutamate to ALA in chloroplasts, was detected in 6803 cell extracts by the conversion of glutamate-1-semialdehyde to ALA and by the inhibition of this reaction by gabaculin.
Full text
PDF



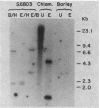
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Avissar Y. J. Biosynthesis of 5-aminolevulinate from glutamate in Anabaena variabilis. Biochim Biophys Acta. 1980;613(1):220–228. doi: 10.1016/0005-2744(80)90208-9. [DOI] [PubMed] [Google Scholar]
- Beale S. I., Gough S. P., Granick S. Biosynthesis of delta-aminolevulinic acid from the intact carbon skeleton of glutamic acid in greening barley. Proc Natl Acad Sci U S A. 1975 Jul;72(7):2719–2723. doi: 10.1073/pnas.72.7.2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brosius J., Dull T. J., Sleeter D. D., Noller H. F. Gene organization and primary structure of a ribosomal RNA operon from Escherichia coli. J Mol Biol. 1981 May 15;148(2):107–127. doi: 10.1016/0022-2836(81)90508-8. [DOI] [PubMed] [Google Scholar]
- Dzelzkalns V. A., Bogorad L. Stable transformation of the cyanobacterium Synechocystis sp. PCC 6803 induced by UV irradiation. J Bacteriol. 1986 Mar;165(3):964–971. doi: 10.1128/jb.165.3.964-971.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray M. W., Doolittle W. F. Has the endosymbiont hypothesis been proven? Microbiol Rev. 1982 Mar;46(1):1–42. doi: 10.1128/mr.46.1.1-42.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guikema J. A., Freeman L., Fleming E. H. Effects of Gabaculine on Pigment Biosynthesis in Normal and Nutrient Deficient Cells of Anacystis nidulans. Plant Physiol. 1986 Sep;82(1):280–284. doi: 10.1104/pp.82.1.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill C. M., Pearson S. A., Smith A. J., Rogers L. J. Inhibition of chlorophyll synthesis in Hordeum vulgare by 3-amino 2,3-dihydrobenzoic acid (gabaculin). Biosci Rep. 1985 Sep;5(9):775–781. doi: 10.1007/BF01119876. [DOI] [PubMed] [Google Scholar]
- Hollingsworth M. J., Hallick R. B. Euglena gracilis chloroplast transfer RNA transcription units. Nucleotide sequence analysis of a tRNATyr-tRNAHis-tRNAMet-tRNATrp-tRNAGlu-tRNAGly gene cluster. J Biol Chem. 1982 Nov 10;257(21):12795–12799. [PubMed] [Google Scholar]
- Huang D. D., Wang W. Y. Chlorophyll biosynthesis in Chlamydomonas starts with the formation of glutamyl-tRNA. J Biol Chem. 1986 Oct 15;261(29):13451–13455. [PubMed] [Google Scholar]
- Huang D. D., Wang W. Y., Gough S. P., Kannangara C. G. delta-Aminolevulinic acid-synthesizing enzymes need an RNA moiety for activity. Science. 1984 Sep 28;225(4669):1482–1484. doi: 10.1126/science.6206568. [DOI] [PubMed] [Google Scholar]
- Höllriegl V., Lamm L., Rowold J., Hörig J., Renz P. Biosynthesis of vitamin B12. Different pathways in some aerobic and anaerobic microorganisms. Arch Microbiol. 1982 Aug;132(2):155–158. doi: 10.1007/BF00508722. [DOI] [PubMed] [Google Scholar]
- Kipe-Nolt J. A., Stevens S. E. Biosynthesis of delta-Aminolevulinic Acid from Glutamate in Agmenellum quadruplicatum. Plant Physiol. 1980 Jan;65(1):126–128. doi: 10.1104/pp.65.1.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer S. M., Beale S. I., Weinstein J. D. Enzymatic conversion of glutamate to delta-aminolevulinic acid in soluble extracts of Euglena gracilis. J Biol Chem. 1987 Sep 15;262(26):12541–12549. [PubMed] [Google Scholar]
- Morgan S., Körner A., Low K. B., Söll D. Regulation of biosynthesis of aminoacyl-tRNA synthetases and of tRNA in Escherichia coli. I. Isolation and characterization of a mutant with elevated levels of tRNAGln 1. J Mol Biol. 1977 Dec 25;117(4):1013–1031. doi: 10.1016/s0022-2836(77)80010-7. [DOI] [PubMed] [Google Scholar]
- Oh-hama T., Seto H., Miyachi S. 13C-NMR evidence of bacteriochlorophyll a formation by the C5 pathway in Chromatium. Arch Biochem Biophys. 1986 Apr;246(1):192–198. doi: 10.1016/0003-9861(86)90463-7. [DOI] [PubMed] [Google Scholar]
- Pearson R. L., Weiss J. F., Kelmers A. D. Improved separation of transfer RNA's on polychlorotrifuoroethylene-supported reversed-phase chromatography columns. Biochim Biophys Acta. 1971 Feb 11;228(3):770–774. doi: 10.1016/0005-2787(71)90748-9. [DOI] [PubMed] [Google Scholar]
- Rando R. R. Mechanism of the irreversible inhibition of gamma-aminobutyric acid-alpha-ketoglutaric acid transaminase by the neutrotoxin gabaculine. Biochemistry. 1977 Oct 18;16(21):4604–4610. doi: 10.1021/bi00640a012. [DOI] [PubMed] [Google Scholar]
- Schön A., Kannangara C. G., Gough S., Söll D. Protein biosynthesis in organelles requires misaminoacylation of tRNA. Nature. 1988 Jan 14;331(6152):187–190. doi: 10.1038/331187a0. [DOI] [PubMed] [Google Scholar]
- Schön A., Krupp G., Gough S., Berry-Lowe S., Kannangara C. G., Söll D. The RNA required in the first step of chlorophyll biosynthesis is a chloroplast glutamate tRNA. Nature. 1986 Jul 17;322(6076):281–284. doi: 10.1038/322281a0. [DOI] [PubMed] [Google Scholar]
- Shinozaki K., Ohme M., Tanaka M., Wakasugi T., Hayashida N., Matsubayashi T., Zaita N., Chunwongse J., Obokata J., Yamaguchi-Shinozaki K. The complete nucleotide sequence of the tobacco chloroplast genome: its gene organization and expression. EMBO J. 1986 Sep;5(9):2043–2049. doi: 10.1002/j.1460-2075.1986.tb04464.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silberklang M., Gillum A. M., RajBhandary U. L. Use of in vitro 32P labeling in the sequence analysis of nonradioactive tRNAs. Methods Enzymol. 1979;59:58–109. doi: 10.1016/0076-6879(79)59072-7. [DOI] [PubMed] [Google Scholar]
- Sprinzl M., Hartmann T., Meissner F., Moll J., Vorderwülbecke T. Compilation of tRNA sequences and sequences of tRNA genes. Nucleic Acids Res. 1987;15 (Suppl):r53–188. doi: 10.1093/nar/15.suppl.r53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanier R. Y., Cohen-Bazire G. Phototrophic prokaryotes: the cyanobacteria. Annu Rev Microbiol. 1977;31:225–274. doi: 10.1146/annurev.mi.31.100177.001301. [DOI] [PubMed] [Google Scholar]
- Vermaas W. F., Williams J. G., Rutherford A. W., Mathis P., Arntzen C. J. Genetically engineered mutant of the cyanobacterium Synechocystis 6803 lacks the photosystem II chlorophyll-binding protein CP-47. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9474–9477. doi: 10.1073/pnas.83.24.9474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHITFELD P. R., MARKHAM R. Natural configuration of the purine nucleotides in ribonucleic acids; chemical hydrolysis of the dinucleoside phosphates. Nature. 1953 Jun 27;171(4365):1151–1152. doi: 10.1038/1711151a0. [DOI] [PubMed] [Google Scholar]
- Wang W. Y., Huang D. D., Stachon D., Gough S. P., Kannangara C. G. Purification, Characterization, and Fractionation of the delta-Aminolevulinic Acid Synthesizing Enzymes from Light-Grown Chlamydomonas reinhardtii Cells. Plant Physiol. 1984 Mar;74(3):569–575. doi: 10.1104/pp.74.3.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein J. D., Beale S. I. Enzymatic conversion of glutamate to delta-aminolevulinate in soluble extracts of the unicellular green alga, Chlorella vulgaris. Arch Biochem Biophys. 1985 Mar;237(2):454–464. doi: 10.1016/0003-9861(85)90299-1. [DOI] [PubMed] [Google Scholar]
- Weinstein J. D., Beale S. I. RNA is required for enzymatic conversion of glutamate to delta-aminolevulinate by extracts of Chlorella vulgaris. Arch Biochem Biophys. 1985 May 15;239(1):87–93. doi: 10.1016/0003-9861(85)90814-8. [DOI] [PubMed] [Google Scholar]
- Weinstein J. D., Beale S. I. Separate physiological roles and subcellular compartments for two tetrapyrrole biosynthetic pathways in Euglena gracilis. J Biol Chem. 1983 Jun 10;258(11):6799–6807. [PubMed] [Google Scholar]
- Woese C. R. Bacterial evolution. Microbiol Rev. 1987 Jun;51(2):221–271. doi: 10.1128/mr.51.2.221-271.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada Y., Ohki M., Ishikura H. The nucleotide sequence of Bacillus subtilis tRNA genes. Nucleic Acids Res. 1983 May 25;11(10):3037–3045. doi: 10.1093/nar/11.10.3037. [DOI] [PMC free article] [PubMed] [Google Scholar]