Abstract
The murine paired box-containing gene Pax1 is required for normal development of the vertebral column, the sternum, and the scapula. Previous studies have shown that three natural Pax1 mouse mutants, the undulated alleles, exhibit phenotypes of different severity in these skeletal elements. Nevertheless, these analyses have not clarified whether the semidominant Undulated short-tail (Uns) mutation, in which the complete Pax1 locus is deleted, represents a null allele. Moreover, the analyses of the classical undulated mutants did not allow a conclusion with respect to haploinsufficiency of Pax1. To address both questions we have created a Pax1 null allele in mice by gene targeting. Surprisingly, the phenotype of this defined mutation exhibits clear differences to that of Uns. This result strongly indicates the contribution of additional gene(s) to the Uns mutant phenotype. Furthermore, the phenotype of mice heterozygous for the null allele demonstrates that Pax1 is haploinsufficient in some though not all skeletal elements which express Pax1 during embryonic development.
In vertebrates the paired box-containing (Pax) genes consist of nine members (Pax1–Pax9) that code for a family of transcription factors (1, 2). The common motif shared by Pax proteins is the paired domain, which consists of 128 amino acids (3) and exerts the DNA binding function (4). The characterization and analysis of mutations affecting Pax genes in mice, humans, and other species have elucidated their important roles in a variety of tissues during embryonic development (5–8).
In the mouse, expression of Pax1 can be detected in sclerotomal cells from embryonic day 8.5 (E8.5) onward (9, 10). As development proceeds, Pax1 transcripts become confined to mesenchymal condensations, which represent the anlagen of the intervertebral discs, and to the perichondria surrounding the cartilage blastemas of the vertebral bodies (10). Pax1 expression starts also in the pharyngeal pouches at E9.5 (11), in the anterior proximal region of the limb buds at E10.0 (12), and in the developing sternum at E13.0 (ref. 9; E.D. and K.I., unpublished observation).
The three classical undulated alleles in the mouse (13–15) harbor mutations of the Pax1 gene (16, 17). In undulated (un), an amino acid exchange at a conserved position of the paired domain of Pax1 causes reduced DNA binding affinity and altered specificity of the mutant protein (16, 18). The undulated extensive (unex) allele carries a deletion that includes the last exon of the Pax1 gene (19). In spite of lacking the last exon, mutant Pax1 mRNA can be detected by RNase protection assay (19). In the Undulated short-tail (Uns) allele, the complete Pax1 locus is deleted (11, 17). In the following, we will refer to un, unex, and Uns, as Pax1un, Pax1un-ex, and Pax1Un-s, respectively.
The phenotypes of the classical undulated alleles have already been analyzed in detail (10–12, 19–21). In general, in all three alleles the vertebral column (10), the pectoral girdle (12), the sternum (19), and the thymus (11) are affected by size reductions and/or malformations. However, phenotypic differences can be observed among the undulated alleles. The Pax1un and Pax1un-ex mutations are regarded to be recessive because severe skeletal malformations are found only in homozygous animals (17). Nevertheless, occasional mild skeletal abnormalities have been described in heterozygotes of both alleles (10, 19, 20). In contrast, the Pax1Un-s mutation is semidominant as heterozygotes exhibit clear skeletal abnormalities including a very short and strongly kinked tail (10). Homozygous Pax1Un-s mice die perinatally displaying the most severe skeletal malformations among the undulated alleles. As the entire Pax1 locus is absent in the semidominant Pax1Un-s mutation, it has been considered to be a null allele, whereas the Pax1un and Pax1un-ex alleles have been regarded to be hypomorphs with weaker phenotypes compared with that of Pax1Un-s. Based mainly on both the semidominant phenotype and the loss of Pax1 in the Pax1Un-s mutation, Pax1 has been discussed to be haploinsufficient. Nevertheless, a Pax1 null phenotype caused solely by the complete loss of Pax1 function remains to be elucidated because it is not known whether the absence of the Pax1 gene alone results in the phenotype of the Pax1Un-s deletion mutant.
To create a defined null allele, we have inactivated the Pax1 gene by gene targeting. To our surprise, we found considerable differences between the phenotypes of the Pax1 knockout and the Pax1Un-s mutant mice. This finding indicates the contribution of additional gene(s) to the Pax1Un-s phenotype. Instead, we show that the Pax1 null phenotype resembles that of the Pax1un and Pax1un-ex alleles. Moreover, based on our detailed analysis of the heterozygous knockout mice, we prove haploinsufficiency of Pax1 in skeletal elements of the vertebral column and the sternum.
MATERIALS AND METHODS
Generation of Pax1 Knockout Mice.
A Pax1 genomic clone was isolated from a 129/Ola genomic cosmid library, and a 5.3-kb genomic HindIII fragment of the 5′ region of Pax1, including exon 1 and 2, was subcloned into pBluescript (Stratagene), designated as pBPax1H. A 1.0-kb XbaI genomic fragment from pBPax1H located downstream of exon 2 was cloned into pTZ19R (Pharmacia Biotech), generating the short arm of the targeting vector. In the next step, a 1.6-kb HindIII-PstI fragment followed by the 3′ adjacent 1.2-kb PstI fragment were inserted to generate the long arm of the targeting vector. The 1.6-kb SalI-XhoI PGKneo cassette was inserted in opposite orientation to that of the Pax1 gene. Finally the 1.8-kb BamHI MC1-HSV-tk cassette was cloned into the targeting vector, now called pPax1tg2. The vector was linearized by ScaI, and 20 μg of the construct were electroporated into 1.6 × 107 R1 embryonic stem (ES) cells (22) at 250 V and 0.5 mF by using a Gene Pulser (Bio-Rad). Stable clones were grown under double selection by using 200 μg/ml G418 and 2 μM gancyclovir in ES cell medium (23). Stable cell lines were tested for homologous recombination by Southern blot analysis by using a 5′ and a 3′ external probe. ES cells from correctly targeted clones were aggregated to morulae derived from CD1 donor mice and implanted into pseudopregnant CD1 female mice. Chimeric males were mated to C57BL/6 females and the offspring was genotyped by Southern blot and PCR analysis. PCR analysis was performed by using two sets of primer pairs in a single reaction (5′-CTCGCCTGCTCACTCCTATCCG-3′, forward primer, and 5′-ATGAGTGCCCATCTTAGTGC-3′, reverse primer of the wild-type Pax1 allele, and 5′-CGTGACTGTAGAGATTGACG-3′, forward primer, and 5′-TGTCGATCAGGATGATCTGG-3′, reverse primer of the targeted Pax1 allele) at 94°C for 1 min, 60°C for 1 min, and 72°C for 1 min in 32 cycles. Homozygous animals were generated by matings between heterozygotes, and newborn animals from generations two to three were used for this study.
Western Blot Analysis.
E13.5 embryos from heterozygote intercrosses were dissected by cutting off the head and the limbs and removing all internal organs. The remainder was trimmed for most optimal enrichment of vertebral column tissue, and protein extracts were generated. Yolk sac material was collected for DNA preparation and subsequent genotyping by PCR. The protein extracts (30 μg) were subjected to Western blot analysis by using reducing SDS/PAGE conditions, as described (24). A Pax1-specific antiserum was generated by conjugating a 15-aa peptide from the C-terminal part of the Pax1 protein (amino acids 327–341; FKHREGTDRKPPSPG) to keyhole limpet hemocyanin (KLH; Pierce). Two rabbits were immunized s.c. each with 400 μg of the KLH-conjugated Pax1-peptide in Freund’s adjuvant. Subsequently, by using 100 μg of the fusion protein in Freund’s adjuvant, the rabbits were boosted in 4-week intervals. The fourth immune serum of one rabbit, designated as 288-IV, was used with 1:250 dilution.
Animals.
The undulated mice were purchased from The Jackson Laboratory. Undulated extensive and Undulated short tail mice were kindly provided by J. L. Cruickshank (Leeds, U.K.) and A. M. Malashenko (Krosnogorsk, Russia), respectively. Mice from the three undulated alleles have been crossed with C57BL/6 mice for more than 12 generations to establish congenic lines.
Skeletal Preparations.
For skeletal preparations we used newborns heterozygous and homozygous for the targeted allele and wild-type littermates that were obtained from heterozygous intercrosses. The newborns were dissected and skeletons were stained with alcian blue for cartilage and alizarin red for bone as described (25).
RESULTS
Generation of a Pax1 Null Mutation.
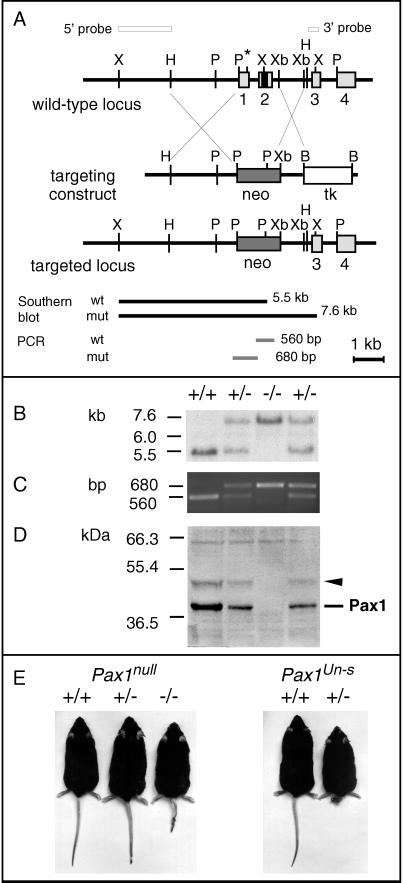
For the purpose of generating a defined Pax1 null mutation, the murine Pax1 gene was disrupted by gene targeting. The targeting strategy was designed to delete the first two coding exons of Pax1 (Fig. 1A). Exon 1 harbors the translational start codon, and exon 2 contains the entire paired box of the Pax1 gene. From 300 stable ES cell clones tested, five proved to be correctly targeted. Male chimeras were generated by morula aggregation from two correctly targeted ES cell lines. Chimeras from one ES cell line passed the mutated allele to the offspring. In these mice, the expected targeting event was confirmed by Southern blot and PCR analyses (Figs. 1 B and C).
Figure 1.
Targeted disruption of the mouse Pax1 gene. (A) Genomic structure of the Pax1 locus and the targeting construct. Light gray colored boxes represent exons 1–4, whereas the black box in exon 2 represents the paired box. The 5′ and 3′ external probes used for the detection of the targeted allele in the Southern blot analysis are shown as open bars. Black bars indicate the fragments detected by Southern blot analysis with the 5′ probe, whereas grey bars indicate the PCR fragments. B, BamHI; H, HindIII; P, PstI; Xb, XbaI; X, XhoI; ∗, start point of translation; tk, MC1-HSV-tk; neo, PGK-neo. (B) Southern blot analysis of genomic DNA from wild-type (+/+), heterozygous (+/−), and homozygous (−/−) Pax1 knockout mice. Liver DNA (10 μg) digested with XhoI was hybridized with the 5′ probe (see A). (C) Genotyping by PCR. The amplification products of the wild-type and mutated allele are depicted in A. (D) Western blot analysis of vertebral column protein extracts from E13.5 embryos. Two bands, the Pax1 protein at 42 kDa and an additional band at 50 kDa (arrowhead), are detected in heterozygous and wild-type control, but not in homozygous protein extracts. (E) Pax1null mice, 9 months old (wild-type control, heterozygous, and homozygous littermates, left to right), and Pax1Un-s mice, 8 months old (wild-type control and heterozygous littermate, left to right). The tail-tip of the Pax1null heterozygous mouse has been clipped.
To prove the absence of Pax1 protein in the mutated mouse line, Western blot analysis was performed. We have generated a polyclonal antiserum directed against the C-terminal part of the Pax1 protein, designated as 288-IV. The Pax1 specificity of this antiserum was demonstrated in Western blot analyses by using protein extracts from Pax1Un-s mutant as well as wild-type control embryos. A 42-kDa band representing the Pax1 protein was detected in wild-type embryos, but was absent in homozygous Pax1Un-s embryos (data not shown). In embryos homozygous for the targeted allele Pax1 protein was not detectable, whereas it appeared at a reduced level in heterozygotes compared with wild-type controls (Fig. 1D). We therefore regard the targeted allele to be a defined null mutation of Pax1, designated as Pax1null. Interestingly, in addition to the authentic Pax1 protein, antiserum 288-IV detected a protein at approximately 50 kDa that was also found to be reduced in heterozygotes and absent in homozygotes (arrowhead, Fig. 1D).
Mice of the expected genotypes from crosses between Pax1null heterozygotes are born at a Mendelian ratio (Table 1). A genetic complementation test between Pax1null and Pax1un mice confirmed allelism (not shown).
Table 1.
Statistics of inheritance and of skeletal phenotypes in newborn animals from crosses between Pax1null heterozygotes
| +/+ | +/− | −/− | |
|---|---|---|---|
| Genotype | |||
| Numbers | 19 | 32 | 20 |
| Ratio | 0.27 | 0.45 | 0.28 |
| Skeletal abnormalities, % | |||
| Atlas-axis | 10 (2) | 63 (20) | 100 (20) |
| Lumbar vertebrae | 16 (3) | 44 (14) | 100 (20) |
| Tail | 0 (0) | 0 (0) | 100 (20) |
| Sternum | 5 (1) | 34 (11) | 100 (20) |
| Scapula | 0 (0) | 0 (0) | 100 (20) |
| Overall | 31 (6) | 88 (28) | 100 (20) |
The percentage of animals with abnormalities in a given skeletal structure are listed in columns corresponding to their genotypes. The numbers of animals with abnormalities are given in parentheses. For the ratio of overall phenotype per genotype, the total number of animals with any of the described malformations was counted. For description of phenotypes, see text.
Pax1null heterozygous mice have an external appearance indistinguishable from that of wild-type littermates, whereas the homozygotes display a strongly kinked tail (Fig. 1E). Though smaller in size than heterozygous and wild-type mice, the Pax1null homozygotes are viable and fertile. Thus, from their appearance, they are similar to homozygous mice of the Pax1un and Pax1un-ex alleles (10), whereas they do not resemble Pax1Un-s mutant mice (Fig. 1E).
Skeletal Abnormalities in Pax1null Homozygous Mice.
Examinations of skeletal preparations from homozygous Pax1null newborn animals showed strong skeletal abnormalities all along the vertebral column (Figs. 2 and 3D), in the sternum, and in the scapula (Fig. 2) with full penetrance (Table 1). In general, skeletal structures affected in Pax1null mutants are the same as those found in mutant mice of the three classical undulated alleles. In spite of these similarities, Pax1null mutants exhibit significant differences to both Pax1un and Pax1Un-s mutant mice with respect to the severity of the skeletal abnormalities (Fig. 3).
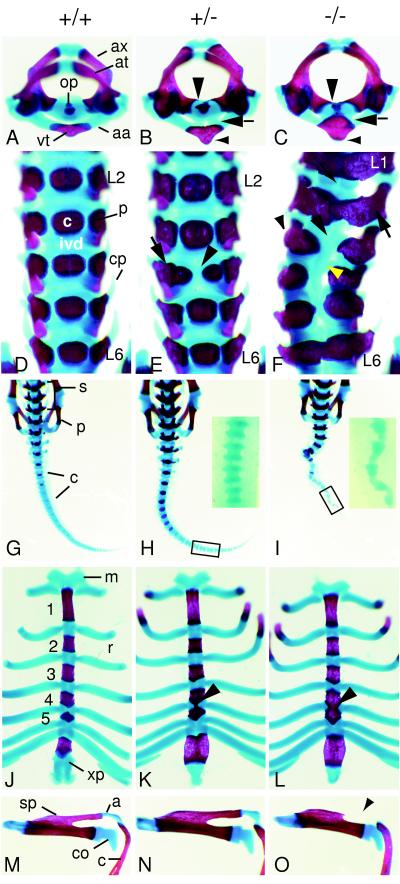
Figure 2.
Skeletal structures of wild-type (A, D, G, J, and M), heterozygous (B, E, H, K, and N), and homozygous (C, F, I, L, and O) Pax1null newborn mice. (A–C) Superior view of atlas (at) and axis (ax). (A) Wild-type newborn. The odontoid process (op) of the axis is clearly separated from the arcus anterior atlantis (aa). vt, Ventral tubercle. (B) In heterozygous animals the ossified ventral tubercle is enlarged (small arrowhead) and is fused to the axis via cartilaginous bridges (arrow). Medial extensions of the pedicles of the axis are indicated by a large arrowhead. (C) In homozygous newborns the ossified ventral tubercle of the arcus anterior atlantis is enlarged (small arrowhead) and fused to the odontoid process of the axis (arrow). The pedicles of the axis extend medially to the ossification center of the vertebral body (large arrowhead). (D–F) Ventral view of lumbar vertebrae. (D) In wild-type newborns the ossification centers of the vertebral bodies (c) are clearly separated from the pedicles (p). Lumbar vertebrae L2 to L6 are shown, each separated by intervertebral discs (ivd). cp, Costal processes. (E) Lumbar vertebrae L2 to L6 of a heterozygous newborn with dual ossification center at L4 (arrowhead) are shown. The right part of the ossification center of L4 is fused to the right pedicle (arrow). (F) Lumbar vertebrae L1 to L6 of homozygous newborn. All lumbar vertebrae display fusions of the pedicles to the ossification centers (large arrow). In L3 to L5 vertebrae are saggitally split, ossification centers are missing (large arrowhead), and costal processes are lacking (small arrowhead). Intervertebral discs are reduced (small arrow), whereas in the sagittally split vertebrae a cartilaginous rod-like structure is formed medially (yellow arrowhead). Note that the malformations lead to skoliosis. (G–I) Dorsal views of sacrum and tail. (G) The sacral (s) and caudal (c) vertebrae of a wild-type newborn animal are shown; p, pelvic girdle. (H) In heterozygotes no difference to wild-type sacrum and tail can be observed. (Inset) A part of the tail (C16–19) in close-up. (I) Homozygous animals exhibit a strongly kinked tail. (Inset) The caudal segments C16–20 are shown in close-up. The vertebrae have a triangular shape caused by a drastically reduced posterior half of the segments. (J–L) Ventral view of the sternum. (J) In wild-type newborns the ossified sternebrae 1–5 are clearly separated by cartilaginous intersternebrae, to which the ribs attach. m, Manubrium; r, rib; xp, xiphoid process. (K) In heterozygotes the cartilaginous intersternebra between sternebrae 4 and 5 frequently exhibits a thin stripe of medio-sagittal ossification (arrowhead). (L) Homozygous newborns show complete ossification of the intersternebra connecting sternebrae 4 and 5 (arrowhead). (M–O) Left scapula in superior view. (M) Wild-type scapula connected to the clavicula (c) via the acromion (a); sp, spine of the scapula; co, coracoid process. (N) Scapula of heterozygote, indistinguishable from that of a wild-type newborn. (O) Scapula from homozygous newborn animal, displaying lack of a part of the ossified spine and of the cartilaginous acromion (arrowhead).
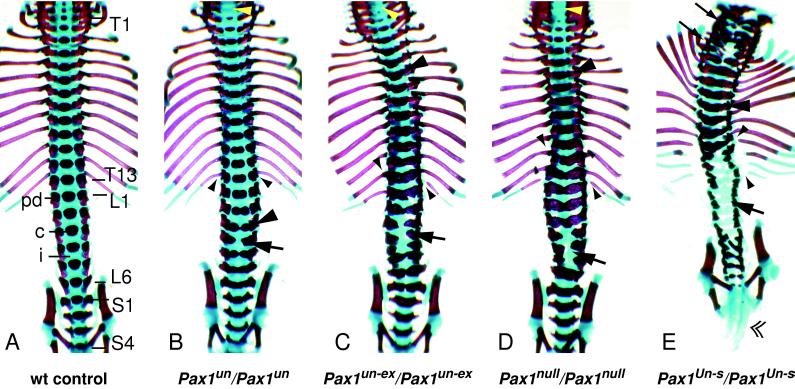
Figure 3.
Ventral view of vertebral columns of Pax1null and the classical undulated homozygous mice at the newborn stage. (A) Wild-type newborn, displaying no phenotypic abnormalities. Vertebral elements from the sixth cervical (C6) to the last sacral (S4) segment are shown. T1–T13, thoracic vertebrae; L1–L6, lumbar vertebrae; S1–S4, sacral vertebrae; pd, pedicle; c, ossification center of vertebral body; i, intervertebral disc. (B) Pax1un homozygote. The ossification center of C7 is missing (yellow arrowhead). Fusions of pedicles to ossification centers at L3 (large arrowhead) and all caudally following segments. Dual ossification centers are found from L2 to L5, and the completely split centrum of L4 is indicated by an arrow. Note that the proximal ends of the 13th rib pair are ossified (small arrowheads). (C) Pax1un-ex homozygote. The ossification center of C5 is missing, and of C6 and C7 are reduced (yellow arrowhead). Fusions of pedicles to ossification centers at T5 (large arrowhead) and all caudally following segments. Vertebrae of the lumbar region (L2 to L4) are saggitally split (arrow). The left 13th rib is not connected to its respective vertebra, and the rib heads of rib pair 12 and the single right rib 13 and left rib 11 are ossified (small arrowheads). (D) Pax1null homozygote with no ossification centers in vertebrae C5 and C6 (yellow arrowhead). Note that pedicles fuse to ossification centers from T5 (large arrowhead) on caudally, and vertebrae of the lumbar region (L3 to L5) are sagittally split (arrow). Note that these malformations lead to skoliosis. The 13th rib pair is not connected to its respective vertebra, and the proximal ends of rib pairs 11 and 12 are ossified (small arrowheads). (E) Pax1Un-s homozygote. Intervertebral discs and vertebral bodies are missing or strongly malformed all along the vertebral column. In addition, in the cervical region vertebrae are ventrally split (small arrows), whereas thoracic vertebrae display fusions between pedicles and ossification centers (large arrowhead). In the lumbar region all ossification centers are missing (large arrow) and the tail is extremely shortened (double arrowhead). The lower ribs are not connected to the vertebral column (small arrowheads).
Axial Skeleton.
In wild-type newborns, the ventral structure of the first cervical vertebra (C1, atlas), the arcus anterior atlantis, is separated from the odontoid process of the second cervical vertebra (C2, axis) by a distinct space (Fig. 2A). In homozygous newborns, this distinct space is not preserved as the odontoid process and the arcus anterior atlantis are fused by ventral cartilaginous and ossified bridges (Fig. 2C, arrow). The pedicles of C2 extend ventro-medially, and the ossified part of the arcus, the ventral tubercle, is enlarged (Fig. 2C, small arrowhead). In addition, in the cervical region ossification centers of the vertebral bodies are reduced or have not formed yet (Fig. 3D, yellow arrowhead). In the thoracic region the pedicles extend ventro-medially, resulting in fusions of the pedicles to the ossification centers of the vertebral bodies. These fusions start from around the fifth thoracic segment (T5) and extend toward the sacral and caudal segments (Fig. 3D). At the thoracic segments T11 to T13 the rib heads (caput costae) are ossified and fused to the pedicles, although a floating 13th rib can also be observed frequently (9/20; Fig. 3D, small arrowheads). Furthermore, the lower thoracic and lumbar vertebrae display an abnormal shape resulting from pronounced fusions of the pedicles across the ventral midline (Figs. 2F and 3D). In the lumbar region we observed saggitally split vertebrae predominantly in L3, L4, and/or L5 (Figs. 2F and 3D). In these segments, intervertebral discs are not formed; instead, a ventral cartilaginous rod-like structure is found (Fig. 2F, yellow arrowhead). Furthermore, the costal processes (Fig. 2F, small arrowhead) and the anapophyses are not formed (data not shown). These abnormalities in the lumbar region can consequently lead to skoliosis (Figs. 2F and 3D). In the tail, the not yet ossified caudal segments are triangular in shape which is caused by a strong reduction of the posterior halves of the vertebrae (Fig. 2I, Inset).
Sternum and Scapula.
The sternum of wild-type newborns displays a regular pattern of ossified sternebrae and cartilaginous intersternebrae to which the ribs attach (Fig. 2J). In homozygous Pax1null newborns sternebrae 4 and 5 are fused, which is caused by complete ossification of the corresponding intersternebra (Fig. 2L, arrowhead). Only a restricted area next to the rib attachment site is chondrified. In few cases the ossification of the intersternebrae extends toward sternebra 3 (data not shown).
In the pectoral girdle the acromion, a chondrified process of the spine of the scapula connects to the humerus and to the clavicle (Fig. 2M). In homozygous Pax1null newborns the acromion process and a part of the spine are not formed (Fig. 2O). In all homozygotes examined both scapulae were always affected to the same extent.
Skeletal Abnormalities in Pax1null Heterozygous Mice.
In 88% of the skeletons from heterozygous Pax1null newborns we observed skeletal abnormalities in the first two cervical vertebrae, in the lumbar region, and/or the sternum (overall ratio, Table 1). In contrast, the tail and the scapulae displayed no malformations in heterozygotes (Table 1; Fig. 2 H and N).
In the majority (63%) of the Pax1null heterozygous newborns, the axis (C2) is ventrally fused to the arcus of the atlas (C1) by cartilaginous bridges (Fig. 2B). These fusions are less pronounced than in homozygous littermates (Fig. 2C). In addition, the ventral tubercle of the arcus is enlarged (Fig. 2B, small arrowhead).
In nearly half (44%) of the heterozygous newborns we found abnormalities of vertebral elements in the lumbar region. In most cases the fourth lumbar vertebra (L4) displays a dual ossification center (Fig. 2E). As a rather rare consequence (3/32), the pedicles fuse to one of the twin ossification centers (Fig. 2E), and costal processes of the affected vertebrae are missing (data not shown).
One-third (34%) of the examined Pax1null heterozygous newborns exhibit a thin stripe of medio-saggital ossification of intersternebra 5 connecting sternebrae 4 and 5 (Fig. 2K). These sternebrae fusions are less severe than those found in homozygous littermates (Fig. 2L).
DISCUSSION
To get clear insight into a defined Pax1 null phenotype and furthermore to clarify whether or not Pax1 is haploinsufficient, we created a Pax1 mutation by gene targeting. Our results show that this knockout allele represents a defined null mutation of Pax1.
Interestingly, the detection of a 50-kDa protein, in addition to the previously described 42-kDa Pax1 protein (18), indicates the presence of a second version of the Pax1 protein generated by some unknown mechanism. The reduction in amount of both the 50-kDa band and the 42-kDa band in Pax1null heterozygotes, and the absence of both in Pax1null homozygotes support this assumption.
Pax1null and the Undulated Alleles.
To our surprise, both heterozygous and homozygous mutants of the Pax1null allele do not resemble the corresponding Pax1Un-s mutant mice in their appearance (Fig. 1E; refs. 10, 17, and 19). As the Pax1null heterozygotes are indistinguishable from wild-type littermates, and the Pax1null homozygotes are viable, they are similar to mutant mice of the Pax1un and Pax1null alleles (10).
Furthermore, from our analysis of the skeletal phenotypes we conclude that Pax1null homozygous mice resemble that of Pax1un and Pax1un-ex homozygotes. Nevertheless, the Pax1un phenotype is less severe than that of the Pax1 null allele, suggesting Pax1un to be a hypomorph (Fig. 3 B and D; refs. 10, 19, and 20). On the other hand, Pax1un-ex and Pax1null mice exhibit virtually identical skeletal phenotypes (Fig. 3 C and D). Therefore we assume that in the Pax1un-ex allele no other gene(s) beside Pax1 are involved in the resulting phenotype, and that Pax1un-ex may be a natural null allele. In contrast, the skeletal phenotype of Pax1Un-s homozygous newborns is much stronger than that of Pax1null homozygotes (Fig. 3 D and E).
In the Pax1Un-s mutation the five coding exons of the Pax1 gene, which are located within a region of 10 kb, are known to be deleted (2, 17). The obvious phenotypic differences we found between the Pax1null and the Pax1Un-s allele suggest that not only Pax1 but also additional gene(s) are involved in the Pax1Un-s mutation. The Pax1Un-s deletion, formerly described to cover at least 48.3 kb (19), is more than 100 kb in size (B.W. and K.I., unpublished observations). We consider the deletion to be large enough to harbor additional gene(s). Interestingly, only Pax1-expressing tissues are affected in Pax1Un-s mutant mice (10). Therefore, we can make certain assumptions about the mechanism of how these additional gene(s) could contribute to the phenotype. For example, a second gene is codeleted which shares expression domains with Pax1 during embryogenesis. Alternatively, as a result of the deletion, a gene located in regions flanking the deletion breakpoints comes under the influence of some distant, yet unknown Pax1 regulatory elements. In this model the second gene could be ectopically activated that in combination with loss of Pax1 would lead to a gain-of-function phenotype in Pax1Un-s. Recently an example for this model has been reported for the Patch (Ph) mutation in which the complete PDGFRα gene is deleted. In Ph, a gene located outside the deletion, c-kit, was found to be ectopically expressed and assumed to contribute to the Ph gain-of-function phenotype (26).
Currently we are performing several approaches to elucidate the molecular basis of the Pax1Un-s mutation. We are in the process of physically mapping the Pax1Un-s deletion breakpoints and in parallel have started to search for genes located in the vicinity of Pax1. Furthermore, experiments to determine the promoter and regulatory elements of the Pax1 gene are underway, as these could not be successfully identified to date. However, preliminary promoter studies suggest that a 3-kb genomic fragment, including the 2-kb region upstream of the first exon, is insufficient to direct specific spatio-temporal Pax1 expression (S.-P. Yee and K.I., unpublished observations). For these purposes, we have established a bacterial artificial chromosome (BAC) contig surrounding the Pax1 locus. By generating mice transgenic for BACs which contain the Pax1 gene we have started rescue experiments for the Pax1null and Pax1Un-s alleles.
Pax1 Is Haploinsufficient.
Although Pax1null heterozygous mice appear externally normal, we demonstrate in this study that almost 90% of the heterozygotes exhibit moderate skeletal malformations in the sternum and parts of the vertebral column. This finding indicates that the Pax1null mutation is semidominant, thus proving haploinsufficiency of Pax1. In contrast, the scapula and the tail are normal in all heterozygotes, showing recessiveness of Pax1null in these structures.
Previous studies on the skeletal phenotypes of Pax1un and Pax1un-ex mutant mice showed weak abnormalities in the sternum and in parts of the vertebral column of heterozygotes (10, 19, 20). Nevertheless, a null phenotype caused solely by the complete loss of Pax1 function could not be defined, and thus haploinsufficiency of Pax1 could not be concluded from these observations.
As not all heterozygous animals are affected to the same extent, the penetrance of the Pax1null heterozygous phenotype seems to be influenced by genetic background effects. On the other hand, the homozygous phenotype shows full penetrance, and the range of variation in phenotypic severity among the homozygotes is much smaller than that among heterozygotes. For comparison, we also have carefully examined wild-type littermates. Less frequently than in heterozygotes, we indeed found recognizable skeletal abnormalities in wild-type controls that, however, in all cases were milder than those observed in heterozygotes.
We could demonstrate that in the developing vertebral column of Pax1null heterozygotes the level of Pax1 protein is reduced. This result, together with the observation of skeletal abnormalities in Pax1null heterozygotes, suggests that Pax1 dosage is critical for the formation of ventral vertebral elements of the cervical and lumbar region, and the sternum.
The Pax1 null mutant described in this report will be an important tool to study genetic interactions between Pax1 and other genes. One of these genes is Pax9 which is highly related to Pax1. Both genes belong to the same subfamily of Pax genes (2, 27) and have a similar sequence, gene structure, and expression pattern (28). Therefore, it is possible that Pax9 might partially substitute for Pax1. The Pax9 gene has recently been inactivated by gene targeting (29). To investigate a potential redundancy between these genes, we are in the process of analyzing double mutants between Pax1 and Pax9 null mice.
Acknowledgments
We thank Drs. M. Hrabé de Angelis, T. Wilm and S.-P. Yee for discussion of the manuscript. We are grateful to U. Huffstadt and S. Bourier for excellent technical assistance with the gene targeting experiment, and U. Linzner (Institute for Pathology/GSF) for the generation of the oligonucleotide primers. This work was in part supported by the Deutsche Forschungsgemeinschaft.
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
Abbreviations: Uns, Undulated short-tail; unex, undulated extensive; un, undulated; E, embryonic day; ES cells, embryonic stem cells.
References
- 1.Walther C, Guenet J-L, Simon D, Deutsch U, Jostes B, Goulding M D, Plachov D, Balling R, Gruss P. Genomics. 1991;11:424–434. doi: 10.1016/0888-7543(91)90151-4. [DOI] [PubMed] [Google Scholar]
- 2.Wallin J, Mizutami Y, Imai K, Miyashita N, Moriwaki K, Taniguchi M, Koseki H, Balling R. Mamm Genome. 1993;4:354–358. doi: 10.1007/BF00360584. [DOI] [PubMed] [Google Scholar]
- 3.Bopp D, Burri M, Baumgartner S, Frigerio G, Noll M. Cell. 1986;47:1033–1040. doi: 10.1016/0092-8674(86)90818-4. [DOI] [PubMed] [Google Scholar]
- 4.Treisman J, Harris E, Desplan C. Genes Dev. 1991;5:594–604. doi: 10.1101/gad.5.4.594. [DOI] [PubMed] [Google Scholar]
- 5.Miskiewicz P, Morrisey D, Lan Y, Raj L, Kessler S, Fujioka M, Goto T, Weir M. Development (Cambridge, UK) 1996;122:2709–2718. doi: 10.1242/dev.122.9.2709. [DOI] [PubMed] [Google Scholar]
- 6.Brand M, Heisenberg C P, Jiang Y J, Beuchle D, Lun K, Furutani-Seiki M, Granato M, Haffter P, Hammerschmidt M, Kane D A, et al. Development (Cambridge, UK) 1996;123:179–190. doi: 10.1242/dev.123.1.179. [DOI] [PubMed] [Google Scholar]
- 7.Fu W, Noll M. Genes Dev. 1997;11:2066–2078. doi: 10.1101/gad.11.16.2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dahl E, Koseki H, Balling R. BioEssays. 1997;19:755–765. doi: 10.1002/bies.950190905. [DOI] [PubMed] [Google Scholar]
- 9.Deutsch U, Dressler G R, Gruss P. Cell. 1988;53:617–625. doi: 10.1016/0092-8674(88)90577-6. [DOI] [PubMed] [Google Scholar]
- 10.Wallin J, Wilting J, Koseki H, Fritsch R, Christ B, Balling R. Development (Cambridge, UK) 1994;120:1109–1121. doi: 10.1242/dev.120.5.1109. [DOI] [PubMed] [Google Scholar]
- 11.Wallin J, Eibel H, Neubüser A, Wilting J, Koseki H, Balling R. Development (Cambridge, UK) 1996;122:23–30. doi: 10.1242/dev.122.1.23. [DOI] [PubMed] [Google Scholar]
- 12.Timmons P M, Wallin J, Rigby P W J, Balling R. Development (Cambridge, UK) 1994;120:2773–2785. doi: 10.1242/dev.120.10.2773. [DOI] [PubMed] [Google Scholar]
- 13.Wright M E. Heredity. 1947;1:137–141. [Google Scholar]
- 14.Blandova Z K, Egorov I K. Mouse News Lett. 1975;52:43. [Google Scholar]
- 15.Wallace M E. J Hered. 1985;76:271–278. doi: 10.1093/oxfordjournals.jhered.a110091. [DOI] [PubMed] [Google Scholar]
- 16.Balling R, Deutsch U, Gruss P. Cell. 1988;55:531–535. doi: 10.1016/0092-8674(88)90039-6. [DOI] [PubMed] [Google Scholar]
- 17.Balling R, Lau C F, Dietrich S, Wallin J, Gruss P. Postimplantation Development in the Mouse. Chichester, U.K.: Wiley; 1992. pp. 132–143. [Google Scholar]
- 18.Chalepakis G, Fritsch R, Fickenscher H, Deutsch U, Goulding M, Gruss P. Cell. 1991;66:873–884. doi: 10.1016/0092-8674(91)90434-z. [DOI] [PubMed] [Google Scholar]
- 19.Dietrich S, Gruss P. Dev Biol. 1995;167:529–548. doi: 10.1006/dbio.1995.1047. [DOI] [PubMed] [Google Scholar]
- 20.Grüneberg H. J Genet. 1950;50:142–173. [PubMed] [Google Scholar]
- 21.Grüneberg H. J Genet. 1954;52:441–455. [Google Scholar]
- 22.Nagy A, Rossant J, Nagy R, Abramow-Newerly W, Roder J C. Proc Natl Acad Sci USA. 1993;90:8424–8428. doi: 10.1073/pnas.90.18.8424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wurst W, Joyner A L. In: Gene Targeting: A Practical Approach. Joyner A L, editor. New York: Oxford Univ. Press; 1993. pp. 33–61. [Google Scholar]
- 24.Peters H, Doll U, Niessing J. Dev Dyn. 1995;203:1–16. doi: 10.1002/aja.1002030102. [DOI] [PubMed] [Google Scholar]
- 25.Kessel M, Balling R, Gruss P. Cell. 1990;61:301–308. doi: 10.1016/0092-8674(90)90810-2. [DOI] [PubMed] [Google Scholar]
- 26.Wehrle-Haller B, Morrison-Graham K, Weston J A. Dev Biol. 1996;177:463–474. doi: 10.1006/dbio.1996.0178. [DOI] [PubMed] [Google Scholar]
- 27.Stapleton P, Weith A, Urbanek P, Kozmik Z, Busslinger M. Nat Genet. 1993;3:292–298. doi: 10.1038/ng0493-292. [DOI] [PubMed] [Google Scholar]
- 28.Neubüser A, Koseki H, Balling R. Dev Biol. 1995;170:701–716. doi: 10.1006/dbio.1995.1248. [DOI] [PubMed] [Google Scholar]
- 29.Peters, H., Neubüser, A., Kratochwil, K. & Balling, R. (1998) Genes Dev., in press. [DOI] [PMC free article] [PubMed]