Abstract
The gene for the extracellular alpha antigen of Mycobacterium bovis BCG was cloned by using a single probe restricted to G or C in the third position. This technique should have great potential for the isolation of mycobacterial antigen genes. The gene analysis revealed that the alpha antigen gene encoded 323 amino acid residues, including 40 amino acids for signal peptide followed by 283 amino acids for mature protein. This is the first report on the structure of the mycobacterial signal peptide. The promoter-like sequence and ribosome-binding site were observed upstream of the open reading frame. In the coding region, the third position of the codon showed high G + C content (86%). The gene was expressed as an unfused protein in Escherichia coli by using an E. coli expression vector. This protein, which reacted with polyclonal antibody raised against alpha antigen from Mycobacterium tuberculosis, would be applicable to the immunodiagnosis of tuberculosis.
Full text
PDF

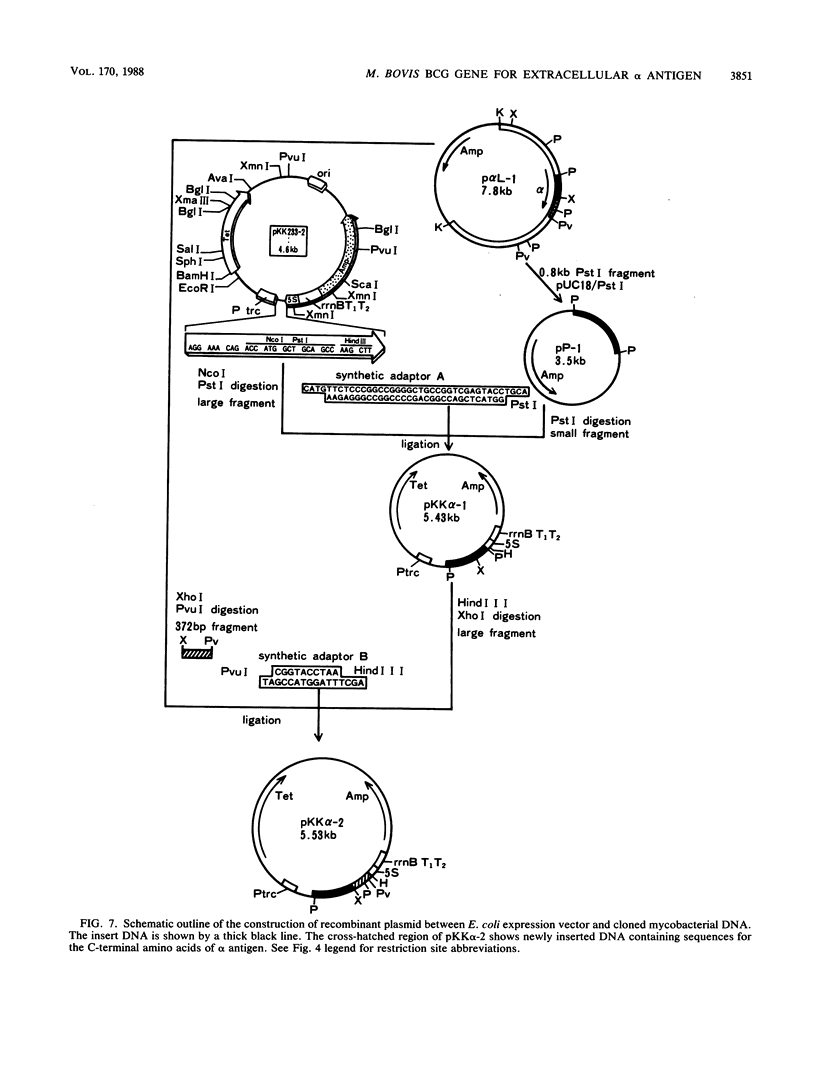
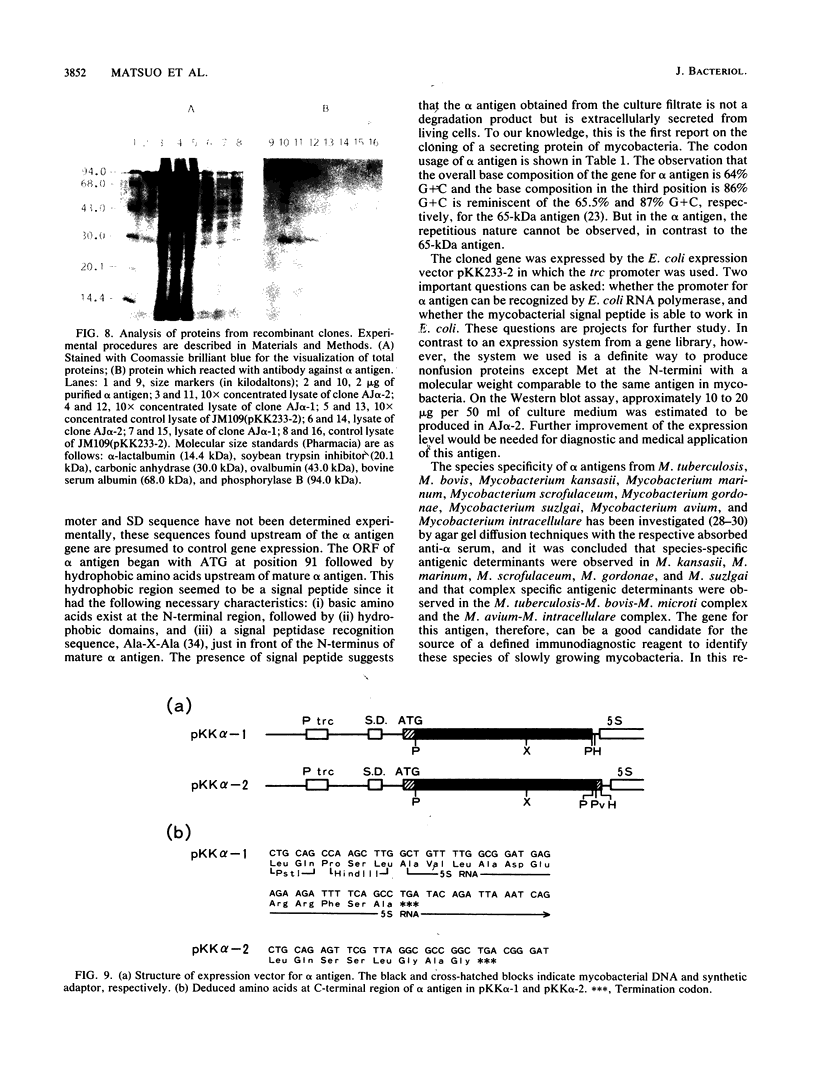
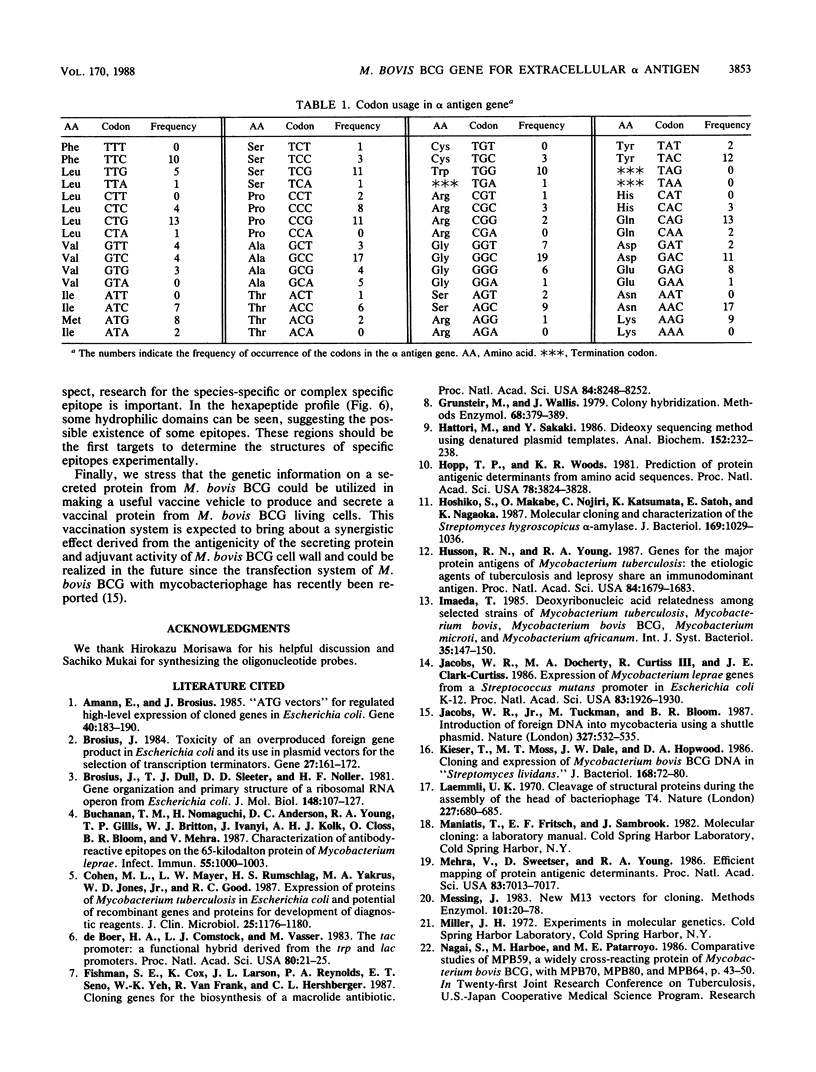

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amann E., Brosius J. "ATG vectors' for regulated high-level expression of cloned genes in Escherichia coli. Gene. 1985;40(2-3):183–190. doi: 10.1016/0378-1119(85)90041-1. [DOI] [PubMed] [Google Scholar]
- Brosius J., Dull T. J., Sleeter D. D., Noller H. F. Gene organization and primary structure of a ribosomal RNA operon from Escherichia coli. J Mol Biol. 1981 May 15;148(2):107–127. doi: 10.1016/0022-2836(81)90508-8. [DOI] [PubMed] [Google Scholar]
- Brosius J. Toxicity of an overproduced foreign gene product in Escherichia coli and its use in plasmid vectors for the selection of transcription terminators. Gene. 1984 Feb;27(2):161–172. doi: 10.1016/0378-1119(84)90137-9. [DOI] [PubMed] [Google Scholar]
- Buchanan T. M., Nomaguchi H., Anderson D. C., Young R. A., Gillis T. P., Britton W. J., Ivanyi J., Kolk A. H., Closs O., Bloom B. R. Characterization of antibody-reactive epitopes on the 65-kilodalton protein of Mycobacterium leprae. Infect Immun. 1987 Apr;55(4):1000–1003. doi: 10.1128/iai.55.4.1000-1003.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen M. L., Mayer L. W., Rumschlag H. S., Yakrus M. A., Jones W. D., Jr, Good R. C. Expression of proteins of Mycobacterium tuberculosis in Escherichia coli and potential of recombinant genes and proteins for development of diagnostic reagents. J Clin Microbiol. 1987 Jul;25(7):1176–1180. doi: 10.1128/jcm.25.7.1176-1180.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishman S. E., Cox K., Larson J. L., Reynolds P. A., Seno E. T., Yeh W. K., Van Frank R., Hershberger C. L. Cloning genes for the biosynthesis of a macrolide antibiotic. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8248–8252. doi: 10.1073/pnas.84.23.8248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunstein M., Wallis J. Colony hybridization. Methods Enzymol. 1979;68:379–389. doi: 10.1016/0076-6879(79)68027-8. [DOI] [PubMed] [Google Scholar]
- Hattori M., Sakaki Y. Dideoxy sequencing method using denatured plasmid templates. Anal Biochem. 1986 Feb 1;152(2):232–238. doi: 10.1016/0003-2697(86)90403-3. [DOI] [PubMed] [Google Scholar]
- Hopp T. P., Woods K. R. Prediction of protein antigenic determinants from amino acid sequences. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3824–3828. doi: 10.1073/pnas.78.6.3824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshiko S., Makabe O., Nojiri C., Katsumata K., Satoh E., Nagaoka K. Molecular cloning and characterization of the Streptomyces hygroscopicus alpha-amylase gene. J Bacteriol. 1987 Mar;169(3):1029–1036. doi: 10.1128/jb.169.3.1029-1036.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husson R. N., Young R. A. Genes for the major protein antigens of Mycobacterium tuberculosis: the etiologic agents of tuberculosis and leprosy share an immunodominant antigen. Proc Natl Acad Sci U S A. 1987 Mar;84(6):1679–1683. doi: 10.1073/pnas.84.6.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs W. R., Docherty M. A., Curtiss R., 3rd, Clark-Curtiss J. E. Expression of Mycobacterium leprae genes from a Streptococcus mutans promoter in Escherichia coli K-12. Proc Natl Acad Sci U S A. 1986 Mar;83(6):1926–1930. doi: 10.1073/pnas.83.6.1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs W. R., Jr, Tuckman M., Bloom B. R. Introduction of foreign DNA into mycobacteria using a shuttle phasmid. Nature. 1987 Jun 11;327(6122):532–535. doi: 10.1038/327532a0. [DOI] [PubMed] [Google Scholar]
- Kieser T., Moss M. T., Dale J. W., Hopwood D. A. Cloning and expression of Mycobacterium bovis BCG DNA in "Streptomyces lividans". J Bacteriol. 1986 Oct;168(1):72–80. doi: 10.1128/jb.168.1.72-80.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mehra V., Sweetser D., Young R. A. Efficient mapping of protein antigenic determinants. Proc Natl Acad Sci U S A. 1986 Sep;83(18):7013–7017. doi: 10.1073/pnas.83.18.7013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messing J. New M13 vectors for cloning. Methods Enzymol. 1983;101:20–78. doi: 10.1016/0076-6879(83)01005-8. [DOI] [PubMed] [Google Scholar]
- Shinnick T. M., Sweetser D., Thole J., van Embden J., Young R. A. The etiologic agents of leprosy and tuberculosis share an immunoreactive protein antigen with the vaccine strain Mycobacterium bovis BCG. Infect Immun. 1987 Aug;55(8):1932–1935. doi: 10.1128/iai.55.8.1932-1935.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinnick T. M. The 65-kilodalton antigen of Mycobacterium tuberculosis. J Bacteriol. 1987 Mar;169(3):1080–1088. doi: 10.1128/jb.169.3.1080-1088.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Suzuki Y., Yoshinaga K., Ono Y., Nagata A., Yamada T. Organization of rRNA genes in Mycobacterium bovis BCG. J Bacteriol. 1987 Feb;169(2):839–843. doi: 10.1128/jb.169.2.839-843.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szostak J. W., Stiles J. I., Tye B. K., Chiu P., Sherman F., Wu R. Hybridization with synthetic oligonucleotides. Methods Enzymol. 1979;68:419–428. doi: 10.1016/0076-6879(79)68031-x. [DOI] [PubMed] [Google Scholar]
- Tasaka H., Kiyotani K., Matsuo Y. Purification and antigenic specificity of alpha protein (Yoneda and Fukui) from Mycobacterium tuberculosis and Mycobacterium intracellulare. Hiroshima J Med Sci. 1983 Mar;32(1):1–8. [PubMed] [Google Scholar]
- Tasaka H., Matsuo Y. Specificity and distribution of alpha antigens of Mycobacterium kansasii and Mycobacterium marinum. Am Rev Respir Dis. 1984 Oct;130(4):647–649. doi: 10.1164/arrd.1984.130.4.647. [DOI] [PubMed] [Google Scholar]
- Tasaka H., Nomura T., Matsuo Y. Specificity and distribution of alpha antigens of Mycobacterium avium-intracellulare, Mycobacterium scrofulaceum, and related species of mycobacteria. Am Rev Respir Dis. 1985 Jul;132(1):173–174. doi: 10.1164/arrd.1985.132.1.173. [DOI] [PubMed] [Google Scholar]
- Thole J. E., Dauwerse H. G., Das P. K., Groothuis D. G., Schouls L. M., van Embden J. D. Cloning of Mycobacterium bovis BCG DNA and expression of antigens in Escherichia coli. Infect Immun. 1985 Dec;50(3):800–806. doi: 10.1128/iai.50.3.800-806.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thole J. E., Keulen W. J., De Bruyn J., Kolk A. H., Groothuis D. G., Berwald L. G., Tiesjema R. H., van Embden J. D. Characterization, sequence determination, and immunogenicity of a 64-kilodalton protein of Mycobacterium bovis BCG expressed in escherichia coli K-12. Infect Immun. 1987 Jun;55(6):1466–1475. doi: 10.1128/iai.55.6.1466-1475.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullrich A., Coussens L., Hayflick J. S., Dull T. J., Gray A., Tam A. W., Lee J., Yarden Y., Libermann T. A., Schlessinger J. Human epidermal growth factor receptor cDNA sequence and aberrant expression of the amplified gene in A431 epidermoid carcinoma cells. 1984 May 31-Jun 6Nature. 309(5967):418–425. doi: 10.1038/309418a0. [DOI] [PubMed] [Google Scholar]
- Wiker H. G., Harboe M., Nagai S., Patarroyo M. E., Ramirez C., Cruz N. MPB59, a widely cross-reacting protein of Mycobacterium bovis BCG. Int Arch Allergy Appl Immunol. 1986;81(4):307–314. doi: 10.1159/000234154. [DOI] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- Yoneda M., Fukui Y., Yamanouchi T. Extracellular proteins of tubercle bacilli. V. Distribution of alpha and beta antigens in various mycobacteria. Biken J. 1965 Dec;8(4):201–223. [PubMed] [Google Scholar]
- Young D. B., Kent L., Young R. A. Screening of a recombinant mycobacterial DNA library with polyclonal antiserum and molecular weight analysis of expressed antigens. Infect Immun. 1987 Jun;55(6):1421–1425. doi: 10.1128/iai.55.6.1421-1425.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young R. A., Bloom B. R., Grosskinsky C. M., Ivanyi J., Thomas D., Davis R. W. Dissection of Mycobacterium tuberculosis antigens using recombinant DNA. Proc Natl Acad Sci U S A. 1985 May;82(9):2583–2587. doi: 10.1073/pnas.82.9.2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Boer H. A., Comstock L. J., Vasser M. The tac promoter: a functional hybrid derived from the trp and lac promoters. Proc Natl Acad Sci U S A. 1983 Jan;80(1):21–25. doi: 10.1073/pnas.80.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Heijne G. How signal sequences maintain cleavage specificity. J Mol Biol. 1984 Feb 25;173(2):243–251. doi: 10.1016/0022-2836(84)90192-x. [DOI] [PubMed] [Google Scholar]