Abstract
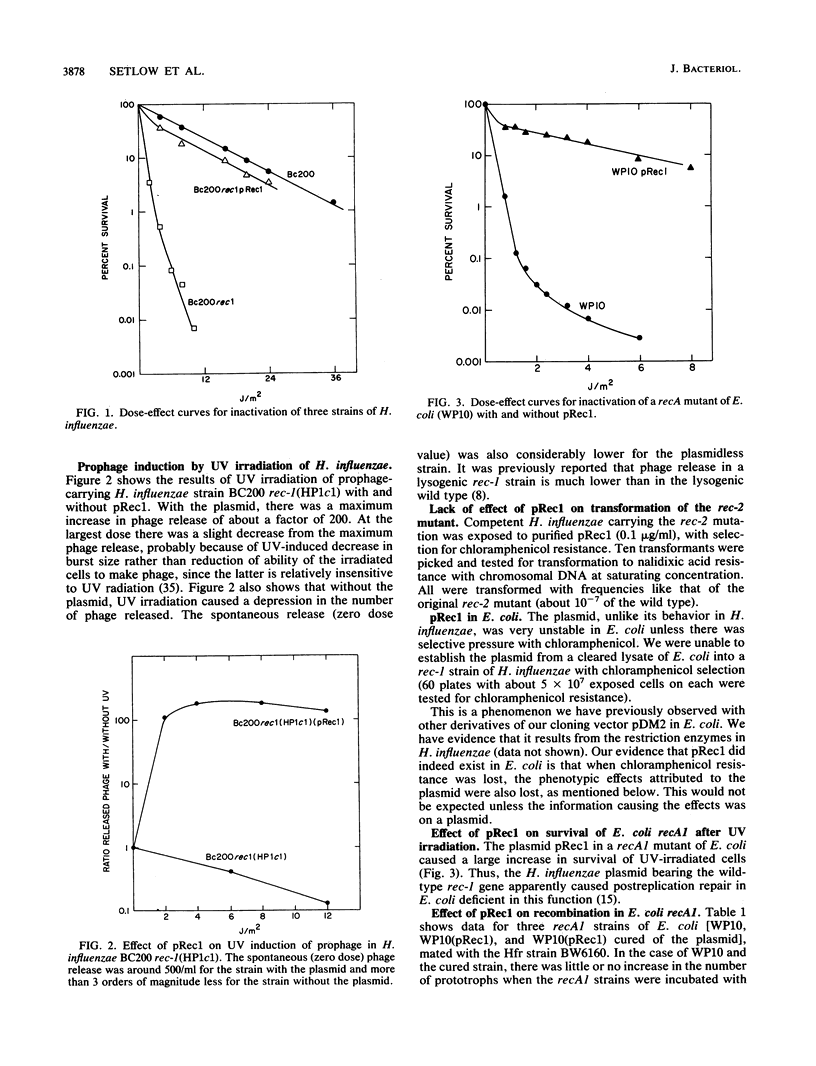
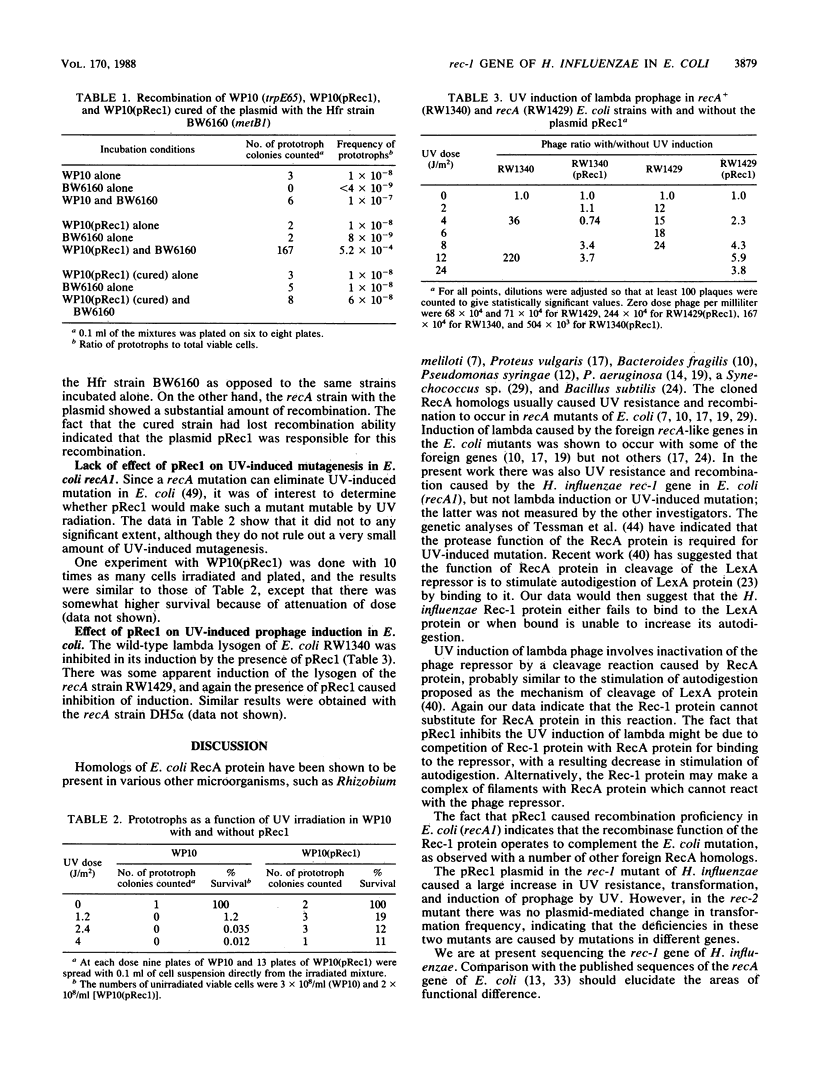
The rec-1 gene of Haemophilus influenzae was cloned into a shuttle vector that replicates in Escherichia coli as well as in H. influenzae. The plasmid, called pRec1, complemented the defects of a rec-1 mutant in repair of UV damage, transformation, and ability of prophage to be induced by UV radiation. Although UV resistance and recombination were caused by pRec1 in E. coli recA mutants, UV induction of lambda and UV mutagenesis were not. We suggest that the ability of the H. influenzae Rec-1 protein to cause cleavage of repressors but not the recombinase function differs from that of the E. coli RecA protein.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balganesh M., Arrigoni L., Setlow J. K. Plasmid-to-chromosome gene transfer in Haemophilus influenza during growth. J Bacteriol. 1986 Oct;168(1):458–459. doi: 10.1128/jb.168.1.458-459.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balganesh M., Setlow J. K. Differential behavior of plasmids containing chromosomal DNA insertions of various sizes during transformation and conjugation in Haemophilus influenzae. J Bacteriol. 1985 Jan;161(1):141–146. doi: 10.1128/jb.161.1.141-146.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balganesh M., Setlow J. K. Genes from plasmid pKM101 in Haemophilus influenzae: separation of functions of mucA and mucB. Proc Natl Acad Sci U S A. 1985 Nov;82(22):7753–7756. doi: 10.1073/pnas.82.22.7753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balganesh M., Setlow J. K. Plasmid-to-plasmid recombination in Haemophilus influenzae. J Bacteriol. 1986 Jan;165(1):308–311. doi: 10.1128/jb.165.1.308-311.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnhart B. J., Cox S. H. Radiation-sensitive and radiation-resistant mutants of Haemophilus influenzae. J Bacteriol. 1968 Jul;96(1):280–282. doi: 10.1128/jb.96.1.280-282.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Better M., Helinski D. R. Isolation and characterization of the recA gene of Rhizobium meliloti. J Bacteriol. 1983 Jul;155(1):311–316. doi: 10.1128/jb.155.1.311-316.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boling M. E., Setlow J. K. Dependence of Vegetative Recombination Among Haemophilus influenzae Bacteriophage on the Host Cell. J Virol. 1969 Sep;4(3):240–243. doi: 10.1128/jvi.4.3.240-243.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark A. J. Recombination deficient mutants of E. coli and other bacteria. Annu Rev Genet. 1973;7:67–86. doi: 10.1146/annurev.ge.07.120173.000435. [DOI] [PubMed] [Google Scholar]
- Goodman H. J., Parker J. R., Southern J. A., Woods D. R. Cloning and expression in Escherichia coli of a recA-like gene from Bacteroides fragilis. Gene. 1987;58(2-3):265–271. doi: 10.1016/0378-1119(87)90381-7. [DOI] [PubMed] [Google Scholar]
- HARM W., RUPERT C. S. INFECTION OF TRANSFORMABLE CELLS OF HAEMOPHILUS INFLUENZAE BY BACTERIOPHAGE AND BACTERIOPHAGE DNA. Z Vererbungsl. 1963 Dec 30;94:336–348. doi: 10.1007/BF00897593. [DOI] [PubMed] [Google Scholar]
- Hickman M. J., Orser C. S., Willis D. K., Lindow S. E., Panopoulos N. J. Molecular cloning and biological characterization of the recA gene from Pseudomonas syringae. J Bacteriol. 1987 Jun;169(6):2906–2910. doi: 10.1128/jb.169.6.2906-2910.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horii T., Ogawa T., Ogawa H. Organization of the recA gene of Escherichia coli. Proc Natl Acad Sci U S A. 1980 Jan;77(1):313–317. doi: 10.1073/pnas.77.1.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn J. M., Ohman D. E. Transcriptional and translational analyses of recA mutant alleles in Pseudomonas aeruginosa. J Bacteriol. 1988 Apr;170(4):1637–1650. doi: 10.1128/jb.170.4.1637-1650.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard-Flanders P., Boyce R. P. DNA repair and genetic recombination: studies on mutants of Escherichia coli defective in these processes. Radiat Res. 1966;(Suppl):156+–156+. [PubMed] [Google Scholar]
- Howard-Flanders P. DNA repair. Annu Rev Biochem. 1968;37:175–200. doi: 10.1146/annurev.bi.37.070168.001135. [DOI] [PubMed] [Google Scholar]
- Keener S. L., McNamee K. P., McEntee K. Cloning and characterization of recA genes froM Proteus vulgaris, Erwinia carotovora, Shigella flexneri, and Escherichia coli B/r. J Bacteriol. 1984 Oct;160(1):153–160. doi: 10.1128/jb.160.1.153-160.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimball R. F., Boling M. E., Perdue S. W. Evidence that UV-inducible error-prone repair is absent in Haemophilus influenzae Rd, with a discussion of the relation to error-prone repair of alkylating-agent damage. Mutat Res. 1977 Aug;44(2):183–196. doi: 10.1016/0027-5107(77)90076-8. [DOI] [PubMed] [Google Scholar]
- Kokjohn T. A., Miller R. V. Molecular cloning and characterization of the recA gene of Pseudomonas aeruginosa PAO. J Bacteriol. 1985 Aug;163(2):568–572. doi: 10.1128/jb.163.2.568-572.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kooistra J., Setlow J. K. Similarity in properties and mapping of three Rec mutants of Haemophilus influenzae. J Bacteriol. 1976 Jul;127(1):327–333. doi: 10.1128/jb.127.1.327-333.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kooistra J., Venema G. Fate of donor deoxyribonucleic acid in a highly transformation-deficient strain of Haemophilus influenzae. J Bacteriol. 1974 Sep;119(3):705–717. doi: 10.1128/jb.119.3.705-717.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leclerc J. E., Setlow J. K. Postreplication repair of ultraviolet damage in Haemophilus influenzae. J Bacteriol. 1972 Jun;110(3):930–934. doi: 10.1128/jb.110.3.930-934.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little J. W. Autodigestion of lexA and phage lambda repressors. Proc Natl Acad Sci U S A. 1984 Mar;81(5):1375–1379. doi: 10.1073/pnas.81.5.1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovett C. M., Jr, Roberts J. W. Purification of a RecA protein analogue from Bacillus subtilis. J Biol Chem. 1985 Mar 25;260(6):3305–3313. [PubMed] [Google Scholar]
- Mandel M., Higa A. Calcium-dependent bacteriophage DNA infection. J Mol Biol. 1970 Oct 14;53(1):159–162. doi: 10.1016/0022-2836(70)90051-3. [DOI] [PubMed] [Google Scholar]
- McCarthy D., Clayton N. L., Setlow J. K. A plasmid cloning vehicle for Haemophilus influenzae and Escherichia coli. J Bacteriol. 1982 Sep;151(3):1605–1607. doi: 10.1128/jb.151.3.1605-1607.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy R. C., Bryant D. A., Porter R. D., de Marsac N. T. Molecular cloning and characterization of the recA gene from the cyanobacterium Synechococcus sp. strain PCC 7002. J Bacteriol. 1987 Jun;169(6):2739–2747. doi: 10.1128/jb.169.6.2739-2747.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Notani N. K., Setlow J. K., McCarthy D., Clayton N. L. Transformation of Haemophilus influenzae by plasmid RSF0885. J Bacteriol. 1981 Dec;148(3):812–816. doi: 10.1128/jb.148.3.812-816.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry K. L., Walker G. C. Identification of plasmid (pKM101)-coded proteins involved in mutagenesis and UV resistance. Nature. 1982 Nov 18;300(5889):278–281. doi: 10.1038/300278a0. [DOI] [PubMed] [Google Scholar]
- Roberts J. W., Roberts C. W., Craig N. L. Escherichia coli recA gene product inactivates phage lambda repressor. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4714–4718. doi: 10.1073/pnas.75.10.4714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sancar A., Stachelek C., Konigsberg W., Rupp W. D. Sequences of the recA gene and protein. Proc Natl Acad Sci U S A. 1980 May;77(5):2611–2615. doi: 10.1073/pnas.77.5.2611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setlow J. K., Beattie K. L., Boling M. E. Expression of a recombination gene on transforming DNA in a recombination-defective Haemophilus influenzae recipient cell. J Mol Biol. 1972 Jul 21;68(2):379–381. doi: 10.1016/0022-2836(72)90219-7. [DOI] [PubMed] [Google Scholar]
- Setlow J. K., Boling M. E., Allison D. P., Beattie K. L. Relationship between prophage induction and transformation in Haemophilus influenzae. J Bacteriol. 1973 Jul;115(1):153–161. doi: 10.1128/jb.115.1.153-161.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setlow J. K., Boling M. E. Bacteriophage of Haemophilus influenzae. II. Repair of ultraviolet-irradiated phage DNA and the capacity of irradiated cells to make phage. J Mol Biol. 1972 Feb 14;63(3):349–362. doi: 10.1016/0022-2836(72)90432-9. [DOI] [PubMed] [Google Scholar]
- Setlow J. K., Boling M. E., Beattie K. L., Kimball R. F. A complex of recombination and repair genes in Haemophilus influenzae. J Mol Biol. 1972 Jul 21;68(2):361–378. doi: 10.1016/0022-2836(72)90218-5. [DOI] [PubMed] [Google Scholar]
- Setlow J. K., Brown D. C., Boling M. E., Mattingly A., Gordon M. P. Repair of deoxyribonucleic acid in Haemophilus influenzae. I. X-ray sensitivity of ultraviolet-sensitive mutants and their behavior as hosts to ultraviolet-irradiated bacteriophage and transforming deoxyribonucleic acid. J Bacteriol. 1968 Feb;95(2):546–558. doi: 10.1128/jb.95.2.546-558.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setlow J. K., Notani N. K., McCarthy D., Clayton N. L. Transformation of Haemophilus influenzae by plasmid RSF0885 containing a cloned segment of chromosomal deoxyribonucleic acid. J Bacteriol. 1981 Dec;148(3):804–811. doi: 10.1128/jb.148.3.804-811.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slilaty S. N., Little J. W. Lysine-156 and serine-119 are required for LexA repressor cleavage: a possible mechanism. Proc Natl Acad Sci U S A. 1987 Jun;84(12):3987–3991. doi: 10.1073/pnas.84.12.3987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small G. D. Mechanism of gap-filling during postreplication repair of ultraviolet damage in Haemophilus influenzae. J Bacteriol. 1975 Oct;124(1):176–181. doi: 10.1128/jb.124.1.176-181.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small G. D., Setlow J. K., Kooistra J., Shapanka R. Lethal effect of mitomycin C on Haemophilus influenzae. J Bacteriol. 1976 Feb;125(2):643–654. doi: 10.1128/jb.125.2.643-654.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinhart W. L., Herriott R. M. Fate of recipient deoxyribonucleic acid during transformation in Haemophilus influenzae. J Bacteriol. 1968 Nov;96(5):1718–1724. doi: 10.1128/jb.96.5.1718-1724.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tessman E. S., Tessman I., Peterson P. K., Forestal J. D. Roles of RecA protease and recombinase activities of Escherichia coli in spontaneous and UV-induced mutagenesis and in Weigle repair. J Bacteriol. 1986 Dec;168(3):1159–1164. doi: 10.1128/jb.168.3.1159-1164.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VOGEL H. J., BONNER D. M. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956 Jan;218(1):97–106. [PubMed] [Google Scholar]
- Waleh N. S., Stocker B. A. Effect of host lex, recA, recF, and uvrD genotypes on the ultraviolet light-protecting and related properties of plasmid R46 in Escherichia coli. J Bacteriol. 1979 Feb;137(2):830–838. doi: 10.1128/jb.137.2.830-838.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker G. C., Dobson P. P. Mutagenesis and repair deficiencies of Escherichia coli umuC mutants are suppressed by the plasmid pKM101. Mol Gen Genet. 1979 Apr 17;172(1):17–24. doi: 10.1007/BF00276210. [DOI] [PubMed] [Google Scholar]
- Willetts N. S., Clark A. J. Characteristics of some multiply recombination-deficient strains of Escherichia coli. J Bacteriol. 1969 Oct;100(1):231–239. doi: 10.1128/jb.100.1.231-239.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkin E. M. The mutability toward ultraviolet light of recombination-deficient strains of Escherichia coli. Mutat Res. 1969 Jul-Aug;8(1):9–14. doi: 10.1016/0027-5107(69)90135-3. [DOI] [PubMed] [Google Scholar]



