Abstract
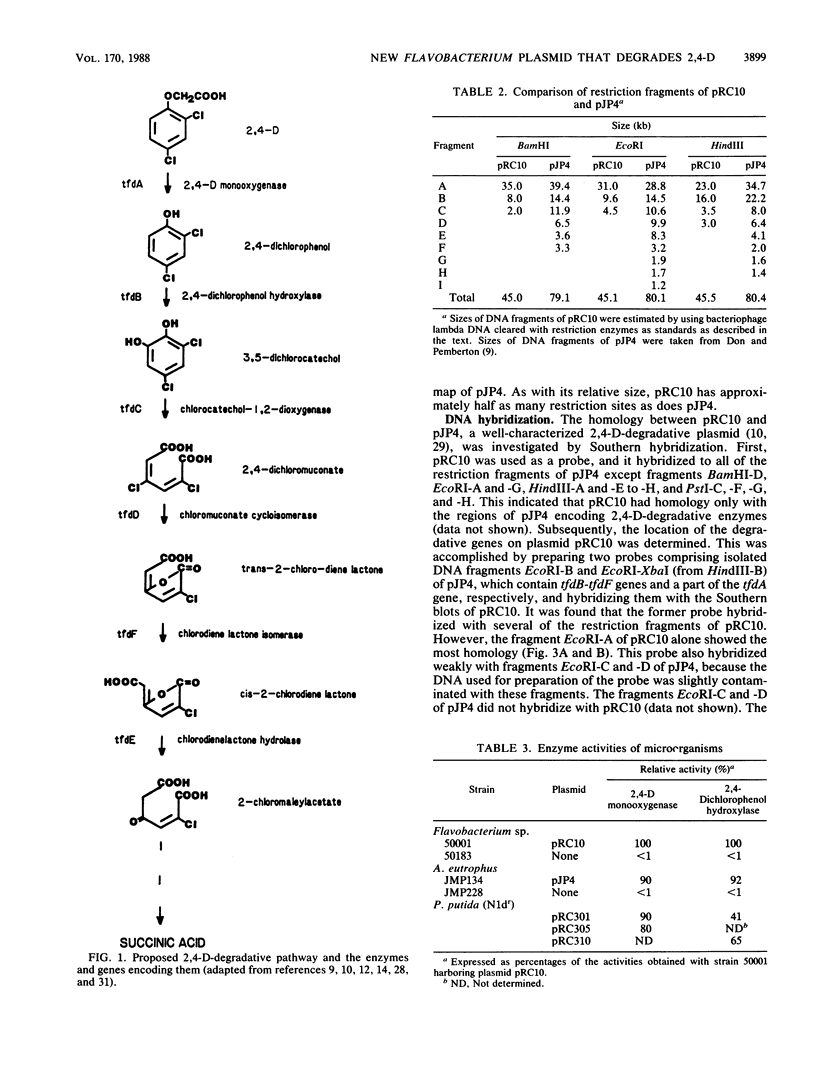
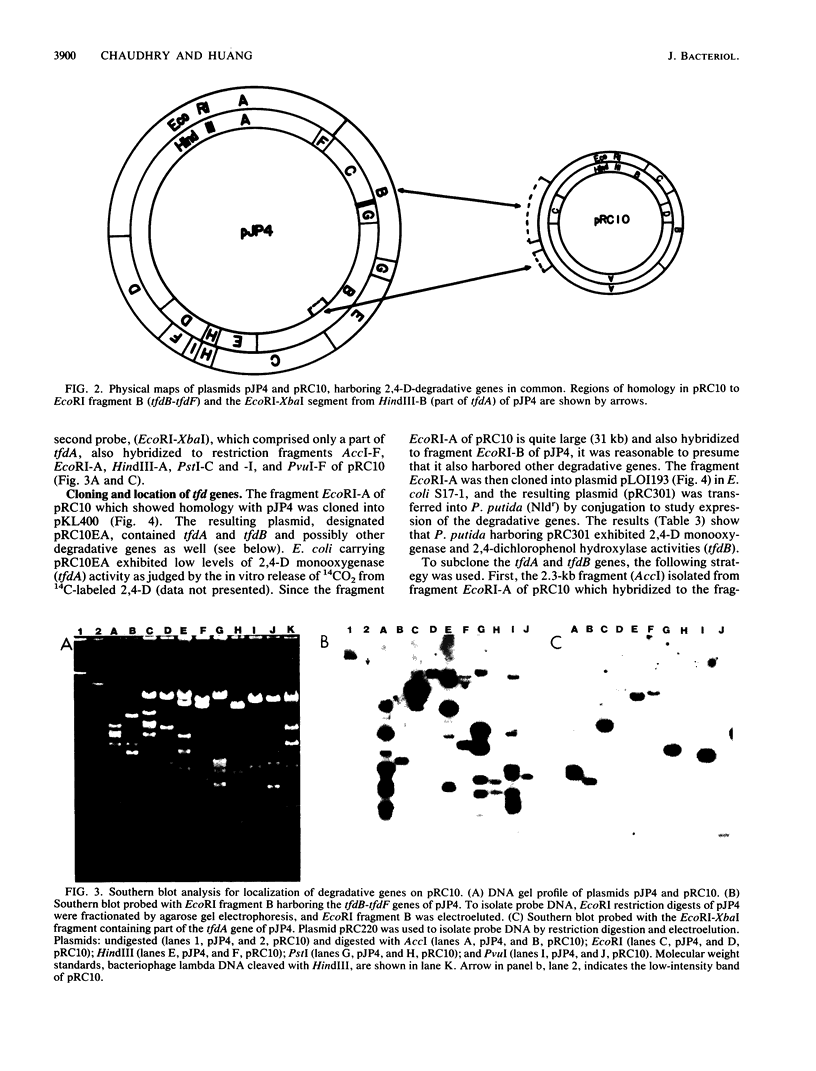
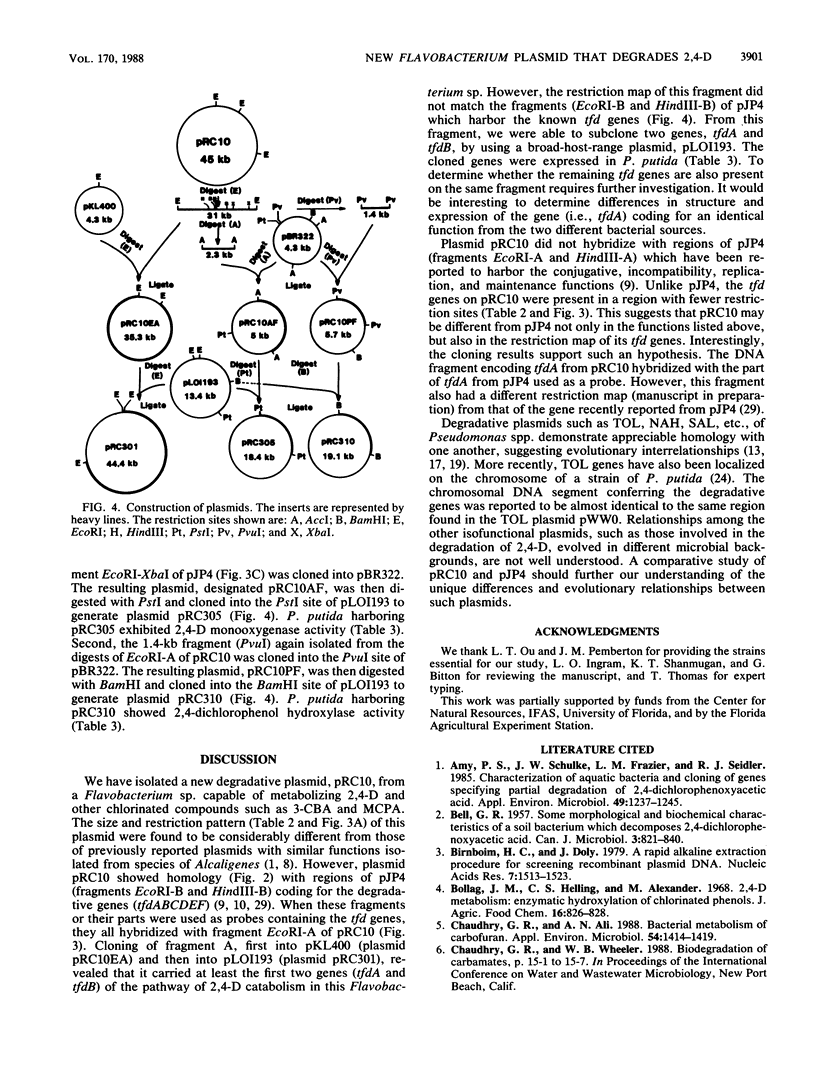
A Flavobacterium sp. (strain 50001), capable of degrading 2,4-dichlorophenoxyacetate (2,4-D), 2-methyl-4-chlorophenoxyacetate, and 2-chlorobenzoate and imparting resistance to mercury, harbored a degradative plasmid, pRC10. Cured strains of the Flavobacterium sp. lost the plasmid as well as the ability to degrade these chlorinated compounds. Comparison of this plasmid with the well-characterized 2,4-D-degradative plasmid pJP4 from Alcaligenes eutrophus showed regions of homology between the two plasmids. Restriction fragments of plasmid pRC10 which shared homology with the regions conferring 2,4-D-degradative genes (tfd) of plasmid pJP4 were cloned into a broad-host-range plasmid and studied in Pseudomonas putida. From the results obtained, the cloned DNA fragment expressed the genes for 2,4-D monooxygenase (tfdA) and 2,4-dichlorophenol hydroxylase (tfdB). In spite of the similarity in function, the size (45 kilobases) and restriction pattern of plasmid pRC10 were considerably different from those of pJP4 (80 kilobases). This may be due to the difference in the microbial background during evolution of the two plasmids.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amy P. S., Schulke J. W., Frazier L. M., Seidler R. J. Characterization of aquatic bacteria and cloning of genes specifying partial degradation of 2,4-dichlorophenoxyacetic acid. Appl Environ Microbiol. 1985 May;49(5):1237–1245. doi: 10.1128/aem.49.5.1237-1245.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BELL G. R. Some morphological and biochemical characteristics of a soil bacterium which decomposes 2, 4-dichlorophenoxyacetic acid. Can J Microbiol. 1957 Oct;3(6):821–840. doi: 10.1139/m57-092. [DOI] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhry G. R., Ali A. N. Bacterial metabolism of carbofuran. Appl Environ Microbiol. 1988 Jun;54(6):1414–1419. doi: 10.1128/aem.54.6.1414-1419.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway T., Byun M. O., Ingram L. O. Expression Vector for Zymomonas mobilis. Appl Environ Microbiol. 1987 Feb;53(2):235–241. doi: 10.1128/aem.53.2.235-241.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Don R. H., Pemberton J. M. Genetic and physical map of the 2,4-dichlorophenoxyacetic acid-degradative plasmid pJP4. J Bacteriol. 1985 Jan;161(1):466–468. doi: 10.1128/jb.161.1.466-468.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Don R. H., Pemberton J. M. Properties of six pesticide degradation plasmids isolated from Alcaligenes paradoxus and Alcaligenes eutrophus. J Bacteriol. 1981 Feb;145(2):681–686. doi: 10.1128/jb.145.2.681-686.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Don R. H., Weightman A. J., Knackmuss H. J., Timmis K. N. Transposon mutagenesis and cloning analysis of the pathways for degradation of 2,4-dichlorophenoxyacetic acid and 3-chlorobenzoate in Alcaligenes eutrophus JMP134(pJP4). J Bacteriol. 1985 Jan;161(1):85–90. doi: 10.1128/jb.161.1.85-90.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn N. W., Gunsalus I. C. Transmissible plasmid coding early enzymes of naphthalene oxidation in Pseudomonas putida. J Bacteriol. 1973 Jun;114(3):974–979. doi: 10.1128/jb.114.3.974-979.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans W. C., Smith B. S., Fernley H. N., Davies J. I. Bacterial metabolism of 2,4-dichlorophenoxyacetate. Biochem J. 1971 May;122(4):543–551. doi: 10.1042/bj1220543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaunt J. K., Evans W. C. Metabolism of 4-chloro-2-methylphenoxyacetate by a soil pseudomonad. Ring-fission, lactonizing and delactonizing enzymes. Biochem J. 1971 May;122(4):533–542. doi: 10.1042/bj1220533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosal D., You I. S., Chatterjee D. K., Chakrabarty A. M. Microbial degradation of halogenated compounds. Science. 1985 Apr 12;228(4696):135–142. doi: 10.1126/science.228.4696.135. [DOI] [PubMed] [Google Scholar]
- Harayama S., Leppik R. A., Rekik M., Mermod N., Lehrbach P. R., Reineke W., Timmis K. N. Gene order of the TOL catabolic plasmid upper pathway operon and oxidation of both toluene and benzyl alcohol by the xylA product. J Bacteriol. 1986 Aug;167(2):455–461. doi: 10.1128/jb.167.2.455-461.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinaru A. L., Duggleby C. J., Broda P. Molecular relationships of degradative plasmids determined by in situ hybridisation of their endonuclease-generated fragments. Mol Gen Genet. 1978 Apr 17;160(3):347–351. doi: 10.1007/BF00332979. [DOI] [PubMed] [Google Scholar]
- Inouye S., Nakazawa A., Nakazawa T. Overproduction of the xylS gene product and activation of the xylDLEGF operon on the TOL plasmid. J Bacteriol. 1987 Aug;169(8):3587–3592. doi: 10.1128/jb.169.8.3587-3592.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lehrbach P. R., McGregor I., Ward J. M., Broda P. Molecular relationships between pseudomonas INC P-9 degradative plasmids TOL, NAH, and SAL. Plasmid. 1983 Sep;10(2):164–174. doi: 10.1016/0147-619x(83)90069-0. [DOI] [PubMed] [Google Scholar]
- Pemberton J. M. Degradative plasmids. Int Rev Cytol. 1983;84:155–183. doi: 10.1016/s0074-7696(08)61017-7. [DOI] [PubMed] [Google Scholar]
- STEENSON T. I., WALKER N. The pathway of breakdown of 2:4-dichloro- and 4-chloro-2-methyl-phenoxyacetic acid by bacteria. J Gen Microbiol. 1957 Feb;16(1):146–155. doi: 10.1099/00221287-16-1-146. [DOI] [PubMed] [Google Scholar]
- Sinclair M. I., Maxwell P. C., Lyon B. R., Holloway B. W. Chromosomal location of TOL plasmid DNA in Pseudomonas putida. J Bacteriol. 1986 Dec;168(3):1302–1308. doi: 10.1128/jb.168.3.1302-1308.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Spooner R. A., Bagdasarian M., Franklin F. C. Activation of the xylDLEGF promoter of the TOL toluene-xylene degradation pathway by overproduction of the xylS regulatory gene product. J Bacteriol. 1987 Aug;169(8):3581–3586. doi: 10.1128/jb.169.8.3581-3586.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spooner R. A., Lindsay K., Franklin F. C. Genetic, functional and sequence analysis of the xylR and xylS regulatory genes of the TOL plasmid pWW0. J Gen Microbiol. 1986 May;132(5):1347–1358. doi: 10.1099/00221287-132-5-1347. [DOI] [PubMed] [Google Scholar]
- Streber W. R., Timmis K. N., Zenk M. H. Analysis, cloning, and high-level expression of 2,4-dichlorophenoxyacetate monooxygenase gene tfdA of Alcaligenes eutrophus JMP134. J Bacteriol. 1987 Jul;169(7):2950–2955. doi: 10.1128/jb.169.7.2950-2955.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler J. E., Finn R. K. Growth rates of a pseudomonad on 2,4-dichlorophenoxyacetic acid and 2,4-dichlorophenol. Appl Microbiol. 1974 Aug;28(2):181–184. doi: 10.1128/am.28.2.181-184.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheelis M. L., Ornston L. N. Genetic control of enzyme induction in the -ketoadipate pathway of Pseudomonas putida: deletion mapping of cat mutations. J Bacteriol. 1972 Feb;109(2):790–795. doi: 10.1128/jb.109.2.790-795.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams P. A., Murray K. Metabolism of benzoate and the methylbenzoates by Pseudomonas putida (arvilla) mt-2: evidence for the existence of a TOL plasmid. J Bacteriol. 1974 Oct;120(1):416–423. doi: 10.1128/jb.120.1.416-423.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worsey M. J., Williams P. A. Metabolism of toluene and xylenes by Pseudomonas (putida (arvilla) mt-2: evidence for a new function of the TOL plasmid. J Bacteriol. 1975 Oct;124(1):7–13. doi: 10.1128/jb.124.1.7-13.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]




