Abstract
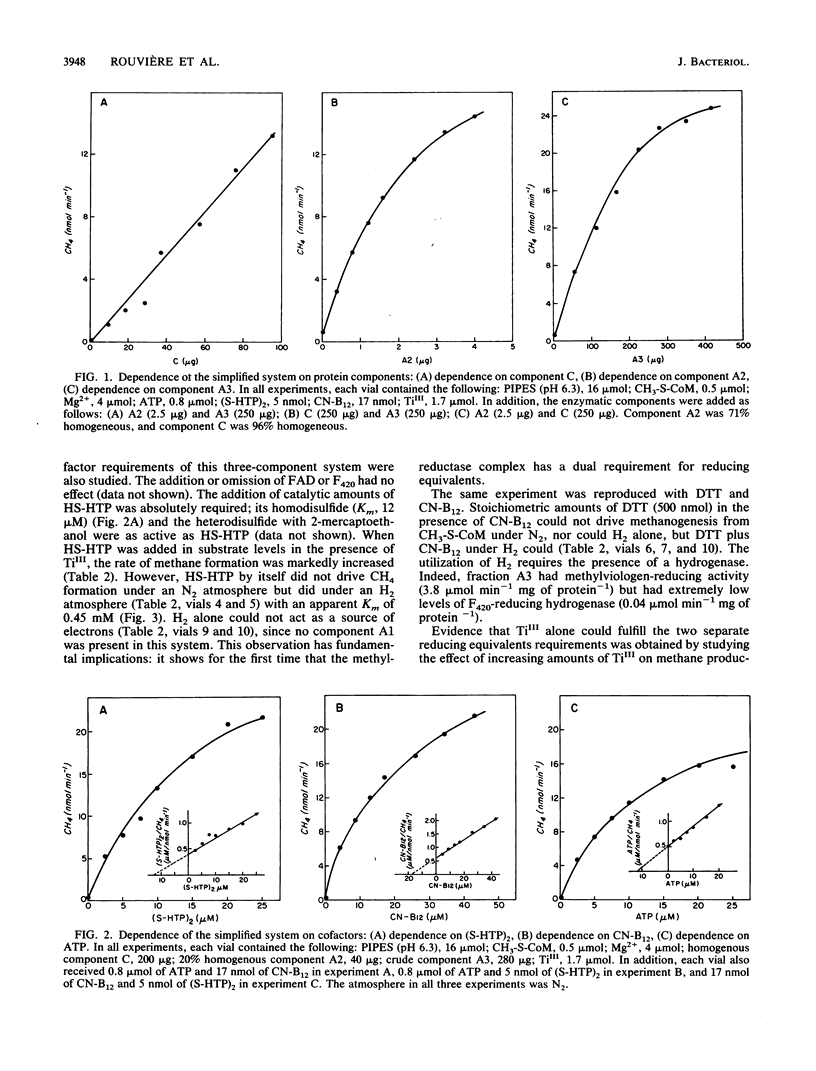
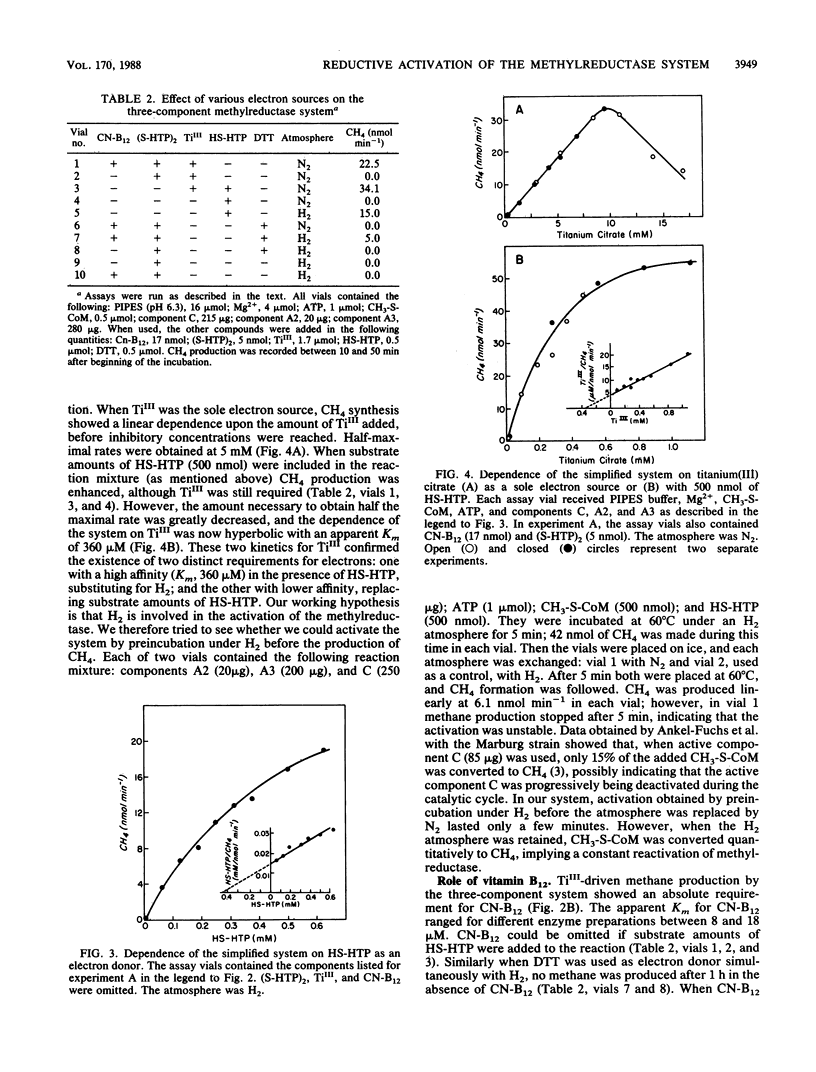
When titanium(III) citrate was used as electron donor for the reduction of methyl coenzyme M by the methyl coenzyme M methylreductase system of Methanobacterium thermoautotrophicum delta H, component A1 was no longer required. The simpler system thus obtained required components A2, A3, and C as well as catalytic amounts of ATP, vitamin B12, and the disulfide of 7-mercaptoheptanoylthreonine phosphate in addition to titanium(III) citrate. This three component enzyme system also could produce CH4 when stoichiometric amounts of 7-mercaptoheptanoylthreonine phosphate were used as a source of electrons under an H2 atmosphere. When 7-mercaptoheptanoylthreonine phosphate or H2 was used alone no CH4 was produced, indicating a dual requirement for reducing equivalents: one to activate the methylreductase system and the other to reduce methyl coenzyme M. This is the first evidence that the activation of methyl coenzyme M methylreductase is a reductive process.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ankel-Fuchs D., Böcher R., Thauer R. K., Noll K. M., Wolfe R. S. 7-Mercaptoheptanoylthreonine phosphate functions as component B in ATP-independent methane formation from methyl-CoM with reduced cobalamin as electron donor. FEBS Lett. 1987 Mar 9;213(1):123–127. doi: 10.1016/0014-5793(87)81476-x. [DOI] [PubMed] [Google Scholar]
- Ankel-Fuchs D., Thauer R. K. Methane formation from methyl-coenzyme M in a system containing methyl-coenzyme M reductase, component B and reduced cobalamin. Eur J Biochem. 1986 Apr 1;156(1):171–177. doi: 10.1111/j.1432-1033.1986.tb09563.x. [DOI] [PubMed] [Google Scholar]
- Bobik T. A., Olson K. D., Noll K. M., Wolfe R. S. Evidence that the heterodisulfide of coenzyme M and 7-mercaptoheptanoylthreonine phosphate is a product of the methylreductase reaction in Methanobacterium. Biochem Biophys Res Commun. 1987 Dec 16;149(2):455–460. doi: 10.1016/0006-291x(87)90389-5. [DOI] [PubMed] [Google Scholar]
- Bobik T. A., Wolfe R. S. Physiological importance of the heterodisulfide of coenzyme M and 7-mercaptoheptanoylthreonine phosphate in the reduction of carbon dioxide to methane in Methanobacterium. Proc Natl Acad Sci U S A. 1988 Jan;85(1):60–63. doi: 10.1073/pnas.85.1.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellefson W. L., Wolfe R. S. Component C of the methylreductase system of Methanobacterium. J Biol Chem. 1981 May 10;256(9):4259–4262. [PubMed] [Google Scholar]
- Ellefson W. L., Wolfe R. S. Role of component C in the methylreductase system of Methanobacterium. J Biol Chem. 1980 Sep 25;255(18):8388–8389. [PubMed] [Google Scholar]
- Ellermann J., Hedderich R., Böcher R., Thauer R. K. The final step in methane formation. Investigations with highly purified methyl-CoM reductase (component C) from Methanobacterium thermoautotrophicum (strain Marburg). Eur J Biochem. 1988 Mar 15;172(3):669–677. doi: 10.1111/j.1432-1033.1988.tb13941.x. [DOI] [PubMed] [Google Scholar]
- Ellermann J., Kobelt A., Pfaltz A., Thauer R. K. On the role of N-7-mercaptoheptanoyl-O-phospho-L-threonine (component B) in the enzymatic reduction of methyl-coenzyme M to methane. FEBS Lett. 1987 Aug 17;220(2):358–362. doi: 10.1016/0014-5793(87)80846-3. [DOI] [PubMed] [Google Scholar]
- Fox J. A., Livingston D. J., Orme-Johnson W. H., Walsh C. T. 8-Hydroxy-5-deazaflavin-reducing hydrogenase from Methanobacterium thermoautotrophicum: 1. Purification and characterization. Biochemistry. 1987 Jul 14;26(14):4219–4227. doi: 10.1021/bi00388a007. [DOI] [PubMed] [Google Scholar]
- Gunsalus R. P., Romesser J. A., Wolfe R. S. Preparation of coenzyme M analogues and their activity in the methyl coenzyme M reductase system of Methanobacterium thermoautotrophicum. Biochemistry. 1978 Jun 13;17(12):2374–2377. doi: 10.1021/bi00605a019. [DOI] [PubMed] [Google Scholar]
- Gunsalus R. P., Tandon S. M., Wolfe R. S. A procedure for anaerobic column chromatography employing an anaerobic Freter-type chamber. Anal Biochem. 1980 Jan 15;101(2):327–331. doi: 10.1016/0003-2697(80)90195-5. [DOI] [PubMed] [Google Scholar]
- Gunsalus R. P., Wolfe R. S. Methyl coenzyme M reductase from Methanobacterium thermoautotrophicum. Resolution and properties of the components. J Biol Chem. 1980 Mar 10;255(5):1891–1895. [PubMed] [Google Scholar]
- Jacobson F. S., Daniels L., Fox J. A., Walsh C. T., Orme-Johnson W. H. Purification and properties of an 8-hydroxy-5-deazaflavin-reducing hydrogenase from Methanobacterium thermoautotrophicum. J Biol Chem. 1982 Apr 10;257(7):3385–3388. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Nagle D. P., Jr, Wolfe R. S. Component A of the methyl coenzyme M methylreductase system of Methanobacterium: resolution into four components. Proc Natl Acad Sci U S A. 1983 Apr;80(8):2151–2155. doi: 10.1073/pnas.80.8.2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noll K. M., Donnelly M. I., Wolfe R. S. Synthesis of 7-mercaptoheptanoylthreonine phosphate and its activity in the methylcoenzyme M methylreductase system. J Biol Chem. 1987 Jan 15;262(2):513–515. [PubMed] [Google Scholar]
- Noll K. M., Rinehart K. L., Jr, Tanner R. S., Wolfe R. S. Structure of component B (7-mercaptoheptanoylthreonine phosphate) of the methylcoenzyme M methylreductase system of Methanobacterium thermoautotrophicum. Proc Natl Acad Sci U S A. 1986 Jun;83(12):4238–4242. doi: 10.1073/pnas.83.12.4238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson A. M., Wolfe R. S. ATP requirement for methanogenesis in cell extracts of methanobacterium strain M.o.H. Biochim Biophys Acta. 1969 Dec 30;192(3):420–429. doi: 10.1016/0304-4165(69)90391-2. [DOI] [PubMed] [Google Scholar]
- Rouvière P. E., Wolfe R. S. 2',3'-Dialdehyde of ATP: a specific, irreversible inhibitor of component A3 of the methylreductase system of Methanobacterium thermoautotrophicum. J Bacteriol. 1987 Apr;169(4):1737–1739. doi: 10.1128/jb.169.4.1737-1739.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stupperich E., Steiner I., Eisinger H. J. Substitution of Co alpha-(5-hydroxybenzimidazolyl)cobamide (factor III) by vitamin B12 in Methanobacterium thermoautotrophicum. J Bacteriol. 1987 Jul;169(7):3076–3081. doi: 10.1128/jb.169.7.3076-3081.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitman W. B., Wolfe R. S. Activation of the methylreductase system from Methanobacterium bryantii by ATP. J Bacteriol. 1983 May;154(2):640–649. doi: 10.1128/jb.154.2.640-649.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitman W. B., Wolfe R. S. Activation of the methylreductase system from Methanobacterium bryantii by corrins. J Bacteriol. 1985 Oct;164(1):165–172. doi: 10.1128/jb.164.1.165-172.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitman W. B., Wolfe R. S. Inhibition by corrins of the ATP-dependent activation and CO2 reduction by the methylreductase system in Methanobacterium bryantii. J Bacteriol. 1987 Jan;169(1):87–92. doi: 10.1128/jb.169.1.87-92.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]



