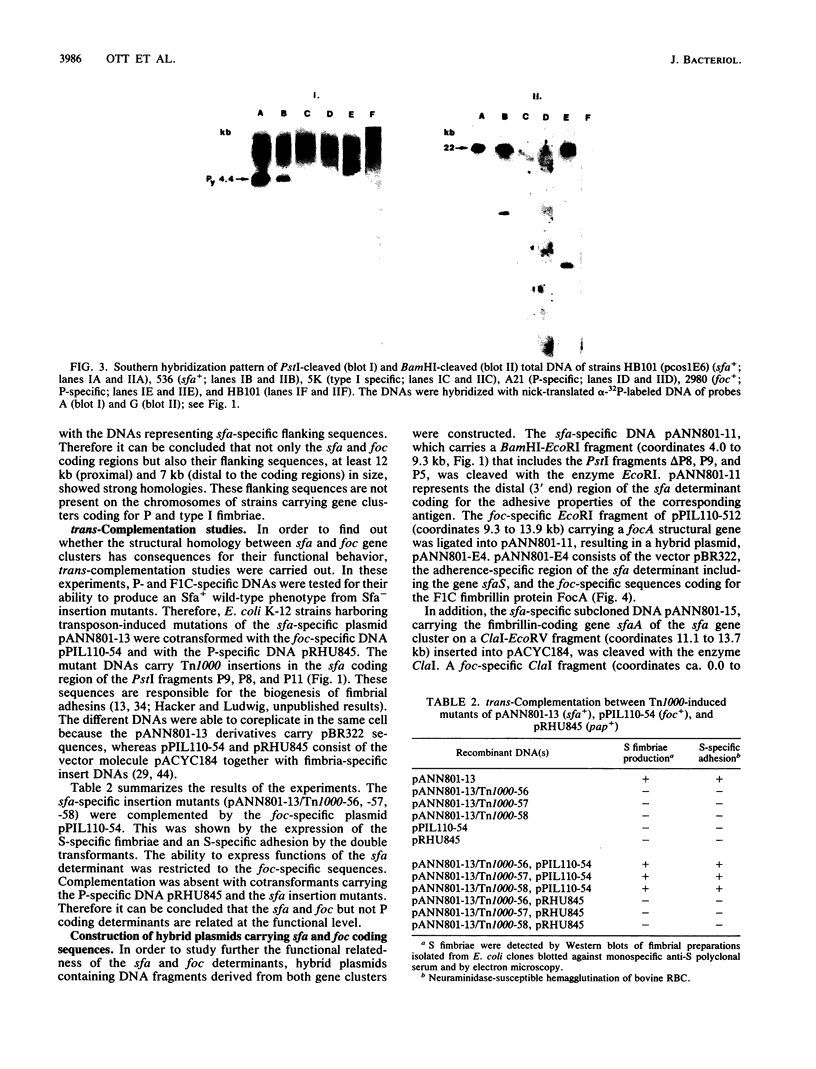
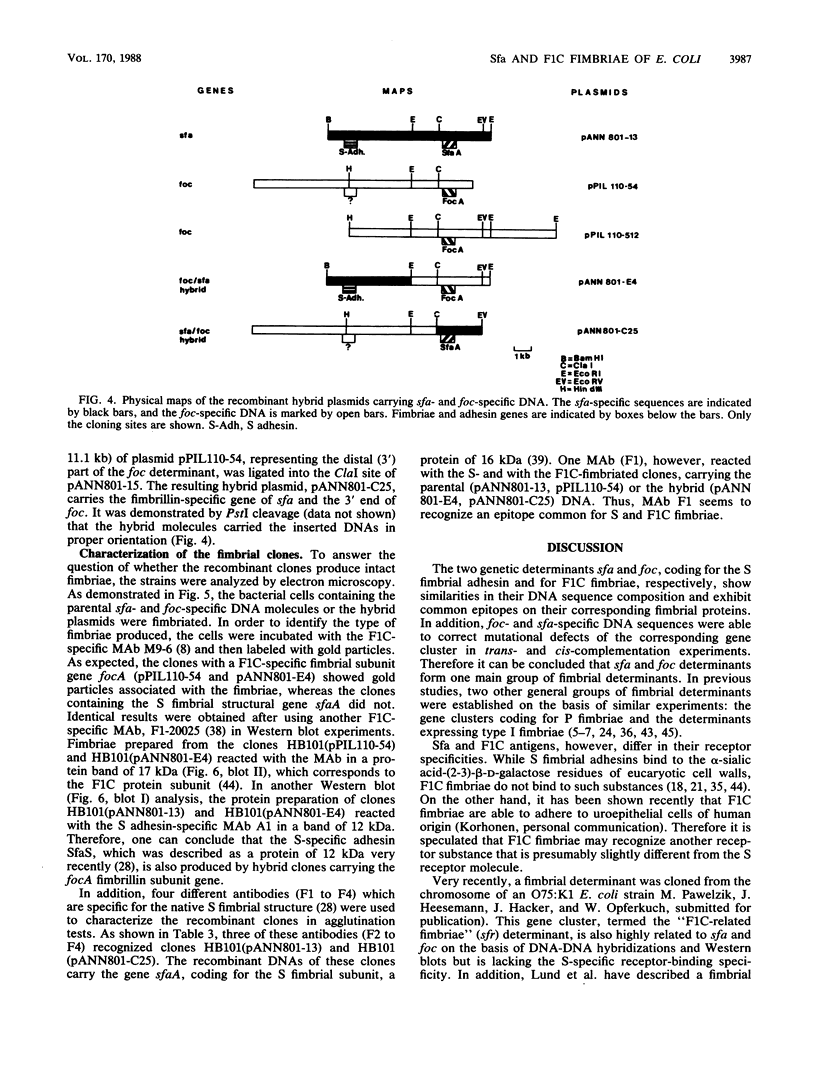
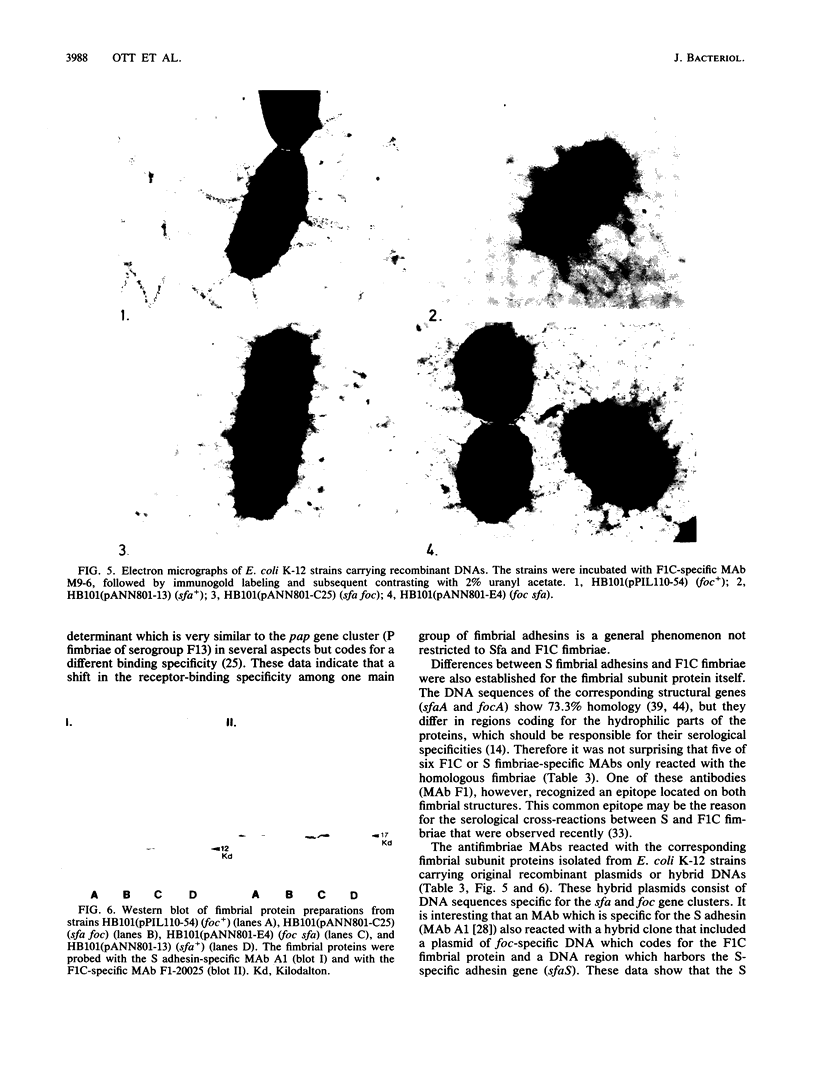
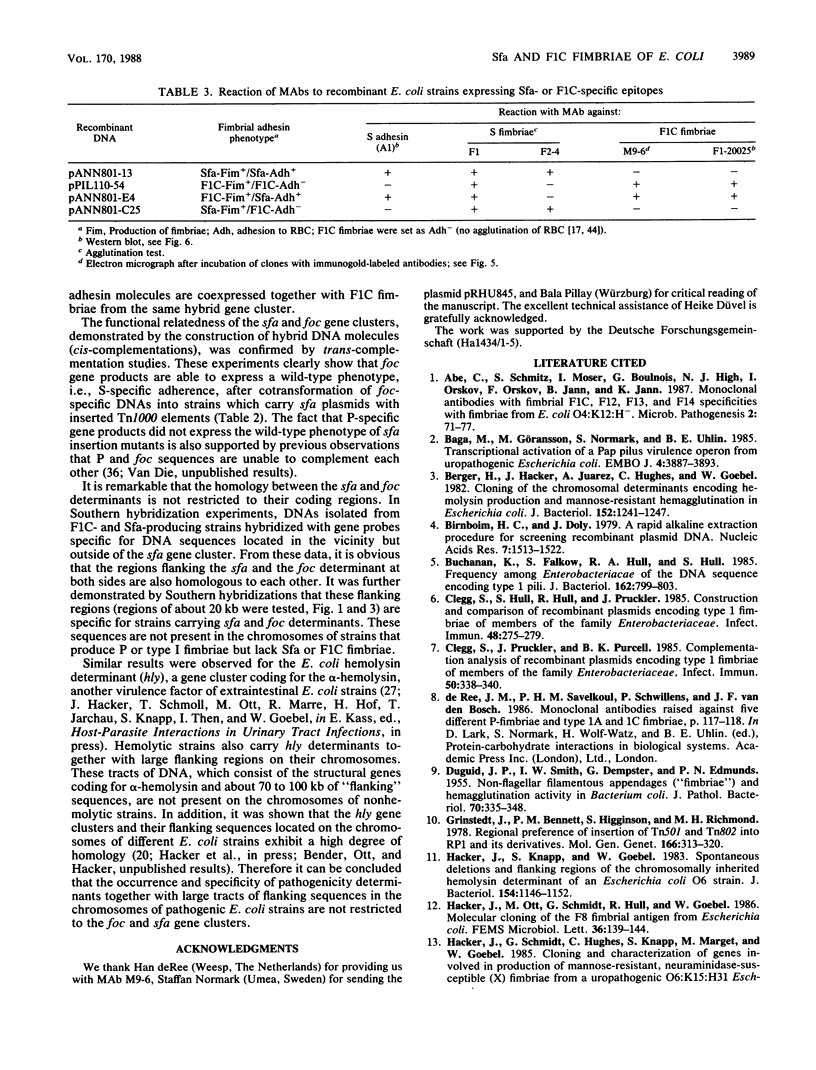
Abstract
Fimbrial adhesins enable bacteria to attach to eucaryotic cells. The genetic determinants for S fimbrial adhesins (sfa) and for F1C ("pseudotype I") fimbriae (foc) were compared. Sfa and F1C represent functionally distinct adhesins in their receptor specificities. Nevertheless, a high degree of homology between both determinants was found on the basis of DNA-DNA hybridizations. Characteristic differences in the restriction maps of the corresponding gene clusters, however, were visible in regions coding for the fimbrial subunits and for the S-specific adhesin. While a plasmid carrying the genetic determinant for F1C fimbriae was able to complement transposon-induced sfa mutants, a plasmid carrying the genetic determinant for a third adhesin type, termed P fimbriae, was unable to do so. Proximal sfa-specific sequences carrying the S fimbrial structural gene were fused to sequences representing the distal part of the foc gene cluster to form a hybrid cluster, and the foc proximal region coding for the structural protein was ligated to sfa distal sequences to form a second hybrid. Both hybrid clones produced intact fimbriae. Anti-F1C monoclonal antibodies (MAbs) only recognized clones which produced F1C fimbriae, and an anti-S adhesin MAb marked clones which expressed the S adhesin. However, one of four other anti-S fimbriae-specific MAbs reacted with both fimbrial structures, S and F1C, indicating a common epitope on both antigens. The results presented here support the view that sfa and foc determinants code for fimbriae that are similar in several aspects, while the P fimbriae are members of a more distantly related group.
Full text
PDF
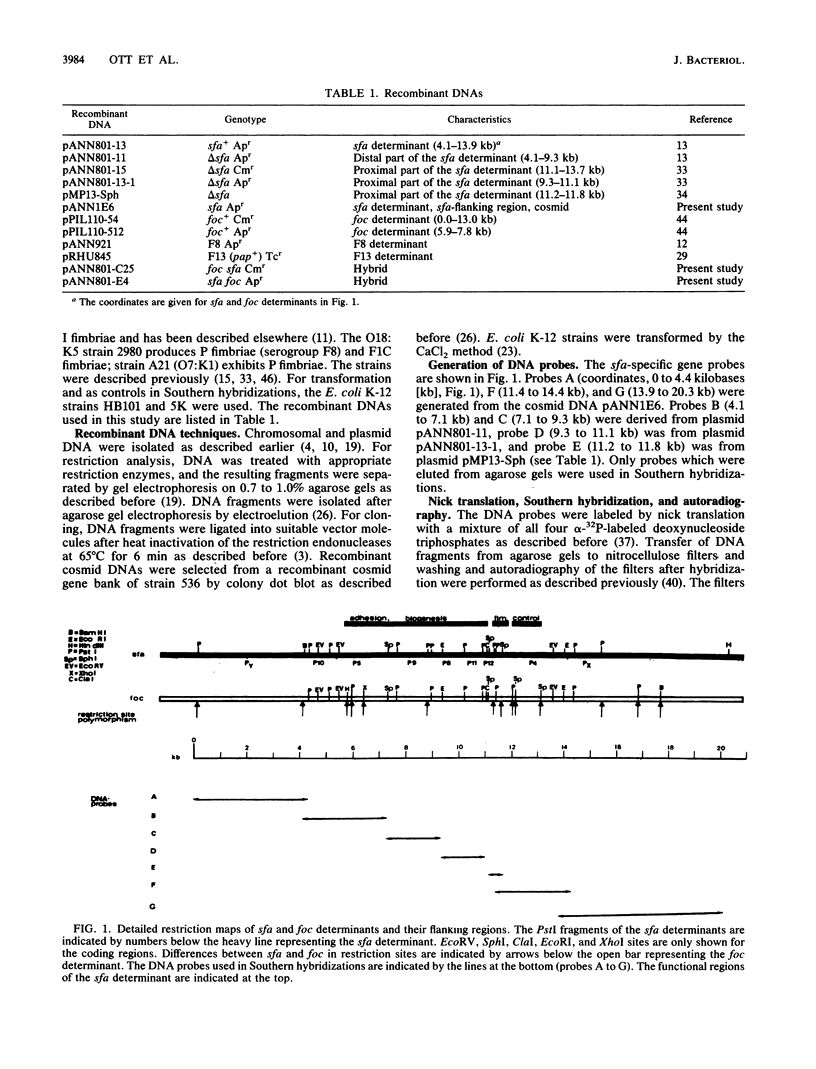


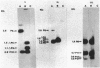
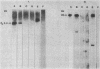
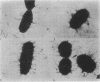
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abe C., Schmitz S., Moser I., Boulnois G., High N. J., Orskov I., Orskov F., Jann B., Jann K. Monoclonal antibodies with fimbrial F1C, F12, F13, and F14 specificities obtained with fimbriae from E. coli 04:K12:H-. Microb Pathog. 1987 Jan;2(1):71–77. doi: 10.1016/0882-4010(87)90116-1. [DOI] [PubMed] [Google Scholar]
- Berger H., Hacker J., Juarez A., Hughes C., Goebel W. Cloning of the chromosomal determinants encoding hemolysin production and mannose-resistant hemagglutination in Escherichia coli. J Bacteriol. 1982 Dec;152(3):1241–1247. doi: 10.1128/jb.152.3.1241-1247.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan K., Falkow S., Hull R. A., Hull S. I. Frequency among Enterobacteriaceae of the DNA sequences encoding type 1 pili. J Bacteriol. 1985 May;162(2):799–803. doi: 10.1128/jb.162.2.799-803.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Båga M., Göransson M., Normark S., Uhlin B. E. Transcriptional activation of a pap pilus virulence operon from uropathogenic Escherichia coli. EMBO J. 1985 Dec 30;4(13B):3887–3893. doi: 10.1002/j.1460-2075.1985.tb04162.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clegg S., Hull S., Hull R., Pruckler J. Construction and comparison of recombinant plasmids encoding type 1 fimbriae of members of the family Enterobacteriaceae. Infect Immun. 1985 May;48(2):275–279. doi: 10.1128/iai.48.2.275-279.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clegg S., Pruckler J., Purcell B. K. Complementation analyses of recombinant plasmids encoding type 1 fimbriae of members of the family Enterobacteriaceae. Infect Immun. 1985 Oct;50(1):338–340. doi: 10.1128/iai.50.1.338-340.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DUGUID J. P., SMITH I. W., DEMPSTER G., EDMUNDS P. N. Non-flagellar filamentous appendages (fimbriae) and haemagglutinating activity in Bacterium coli. J Pathol Bacteriol. 1955 Oct;70(2):335–348. doi: 10.1002/path.1700700210. [DOI] [PubMed] [Google Scholar]
- Grinsted J., Bennett P. M., Higginson S., Richmond M. H. Regional preference of insertion of Tn501 and Tn802 into RP1 and its derivatives. Mol Gen Genet. 1978 Nov 9;166(3):313–320. doi: 10.1007/BF00267624. [DOI] [PubMed] [Google Scholar]
- Hacker J., Knapp S., Goebel W. Spontaneous deletions and flanking regions of the chromosomally inherited hemolysin determinant of an Escherichia coli O6 strain. J Bacteriol. 1983 Jun;154(3):1145–1152. doi: 10.1128/jb.154.3.1145-1152.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopp T. P., Woods K. R. Prediction of protein antigenic determinants from amino acid sequences. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3824–3828. doi: 10.1073/pnas.78.6.3824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klemm P. Fimbrial adhesions of Escherichia coli. Rev Infect Dis. 1985 May-Jun;7(3):321–340. doi: 10.1093/clinids/7.3.321. [DOI] [PubMed] [Google Scholar]
- Klemm P., Orskov I., Orskov F. F7 and type 1-like fimbriae from three Escherichia coli strains isolated from urinary tract infections: protein chemical and immunological aspects. Infect Immun. 1982 May;36(2):462–468. doi: 10.1128/iai.36.2.462-468.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapp S., Hacker J., Jarchau T., Goebel W. Large, unstable inserts in the chromosome affect virulence properties of uropathogenic Escherichia coli O6 strain 536. J Bacteriol. 1986 Oct;168(1):22–30. doi: 10.1128/jb.168.1.22-30.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapp S., Hacker J., Then I., Müller D., Goebel W. Multiple copies of hemolysin genes and associated sequences in the chromosomes of uropathogenic Escherichia coli strains. J Bacteriol. 1984 Sep;159(3):1027–1033. doi: 10.1128/jb.159.3.1027-1033.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korhonen T. K., Valtonen M. V., Parkkinen J., Väisänen-Rhen V., Finne J., Orskov F., Orskov I., Svenson S. B., Mäkelä P. H. Serotypes, hemolysin production, and receptor recognition of Escherichia coli strains associated with neonatal sepsis and meningitis. Infect Immun. 1985 May;48(2):486–491. doi: 10.1128/iai.48.2.486-491.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korhonen T. K., Väisänen-Rhen V., Rhen M., Pere A., Parkkinen J., Finne J. Escherichia coli fimbriae recognizing sialyl galactosides. J Bacteriol. 1984 Aug;159(2):762–766. doi: 10.1128/jb.159.2.762-766.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lederberg E. M., Cohen S. N. Transformation of Salmonella typhimurium by plasmid deoxyribonucleic acid. J Bacteriol. 1974 Sep;119(3):1072–1074. doi: 10.1128/jb.119.3.1072-1074.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund B., Lindberg F. P., Båga M., Normark S. Globoside-specific adhesins of uropathogenic Escherichia coli are encoded by similar trans-complementable gene clusters. J Bacteriol. 1985 Jun;162(3):1293–1301. doi: 10.1128/jb.162.3.1293-1301.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund B., Marklund B. I., Strömberg N., Lindberg F., Karlsson K. A., Normark S. Uropathogenic Escherichia coli can express serologically identical pili of different receptor binding specificities. Mol Microbiol. 1988 Mar;2(2):255–263. doi: 10.1111/j.1365-2958.1988.tb00027.x. [DOI] [PubMed] [Google Scholar]
- Marre R., Hacker J., Henkel W., Goebel W. Contribution of cloned virulence factors from uropathogenic Escherichia coli strains to nephropathogenicity in an experimental rat pyelonephritis model. Infect Immun. 1986 Dec;54(3):761–767. doi: 10.1128/iai.54.3.761-767.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moch T., Hoschützky H., Hacker J., Kröncke K. D., Jann K. Isolation and characterization of the alpha-sialyl-beta-2,3-galactosyl-specific adhesin from fimbriated Escherichia coli. Proc Natl Acad Sci U S A. 1987 May;84(10):3462–3466. doi: 10.1073/pnas.84.10.3462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Normark S., Lark D., Hull R., Norgren M., Båga M., O'Hanley P., Schoolnik G., Falkow S. Genetics of digalactoside-binding adhesin from a uropathogenic Escherichia coli strain. Infect Immun. 1983 Sep;41(3):942–949. doi: 10.1128/iai.41.3.942-949.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowicki B., Vuopio-Varkila J., Viljanen P., Korhonen T. K., Mäkelä P. H. Fimbrial phase variation and systemic E. coli infection studied in the mouse peritonitis model. Microb Pathog. 1986 Aug;1(4):335–347. doi: 10.1016/0882-4010(86)90066-5. [DOI] [PubMed] [Google Scholar]
- Orskov I., Orskov F. Escherichia coli in extra-intestinal infections. J Hyg (Lond) 1985 Dec;95(3):551–575. doi: 10.1017/s0022172400060678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orskov I., Orskov F. Serology of Escherichia coli fimbriae. Prog Allergy. 1983;33:80–105. [PubMed] [Google Scholar]
- Ott M., Hacker J., Schmoll T., Jarchau T., Korhonen T. K., Goebel W. Analysis of the genetic determinants coding for the S-fimbrial adhesin (sfa) in different Escherichia coli strains causing meningitis or urinary tract infections. Infect Immun. 1986 Dec;54(3):646–653. doi: 10.1128/iai.54.3.646-653.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ott M., Schmoll T., Goebel W., Van Die I., Hacker J. Comparison of the genetic determinant coding for the S-fimbrial adhesin (sfa) of Escherichia coli to other chromosomally encoded fimbrial determinants. Infect Immun. 1987 Aug;55(8):1940–1943. doi: 10.1128/iai.55.8.1940-1943.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkkinen J., Rogers G. N., Korhonen T., Dahr W., Finne J. Identification of the O-linked sialyloligosaccharides of glycophorin A as the erythrocyte receptors for S-fimbriated Escherichia coli. Infect Immun. 1986 Oct;54(1):37–42. doi: 10.1128/iai.54.1.37-42.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhen M., Väisänen-Rhen V., Pere A., Korhonen T. K. Complementation and regulatory interaction between two cloned fimbrial gene clusters of Escherichia coli strain KS71. Mol Gen Genet. 1985;200(1):60–64. doi: 10.1007/BF00383312. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Schmitz S., Abe C., Moser I., Orskov I., Orskov F., Jann B., Jann K. Monoclonal antibodies against the nonhemagglutinating fimbrial antigen 1C (pseudotype 1) of Escherichia coli. Infect Immun. 1986 Jan;51(1):54–59. doi: 10.1128/iai.51.1.54-59.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Die I., Van Megen I., Zuidweg E., Hoekstra W., De Ree H., Van den Bosch H., Bergmans H. Functional relationship among the gene clusters encoding F7(1), F7(2), F9, and F11 fimbriae of human uropathogenic Escherichia coli. J Bacteriol. 1986 Jul;167(1):407–410. doi: 10.1128/jb.167.1.407-410.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wevers P., Picken R., Schmidt G., Jann B., Jann K., Golecki J. R., Kist M. Characterization of pili associated with Escherichia coli O18ac. Infect Immun. 1980 Aug;29(2):685–691. doi: 10.1128/iai.29.2.685-691.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Die I., van Geffen B., Hoekstra W., Bergmans H. Type 1C fimbriae of a uropathogenic Escherichia coli strain: cloning and characterization of the genes involved in the expression of the 1C antigen and nucleotide sequence of the subunit gene. Gene. 1985;34(2-3):187–196. doi: 10.1016/0378-1119(85)90127-1. [DOI] [PubMed] [Google Scholar]