Abstract
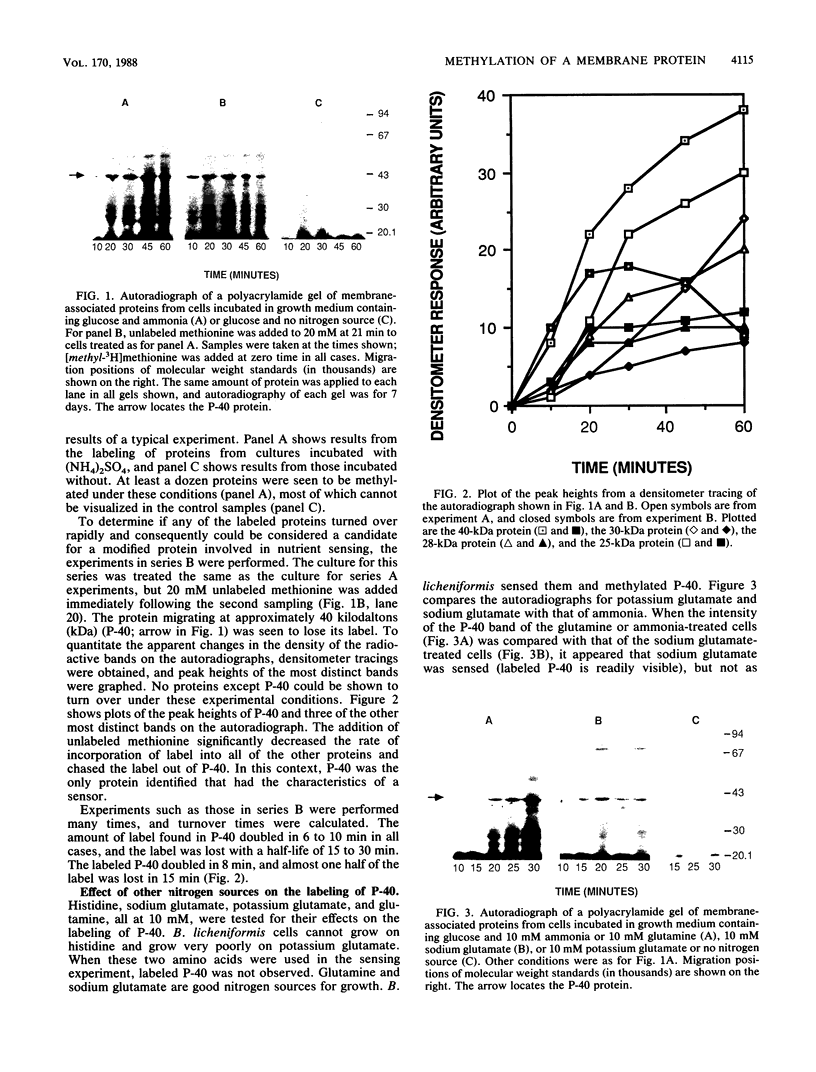
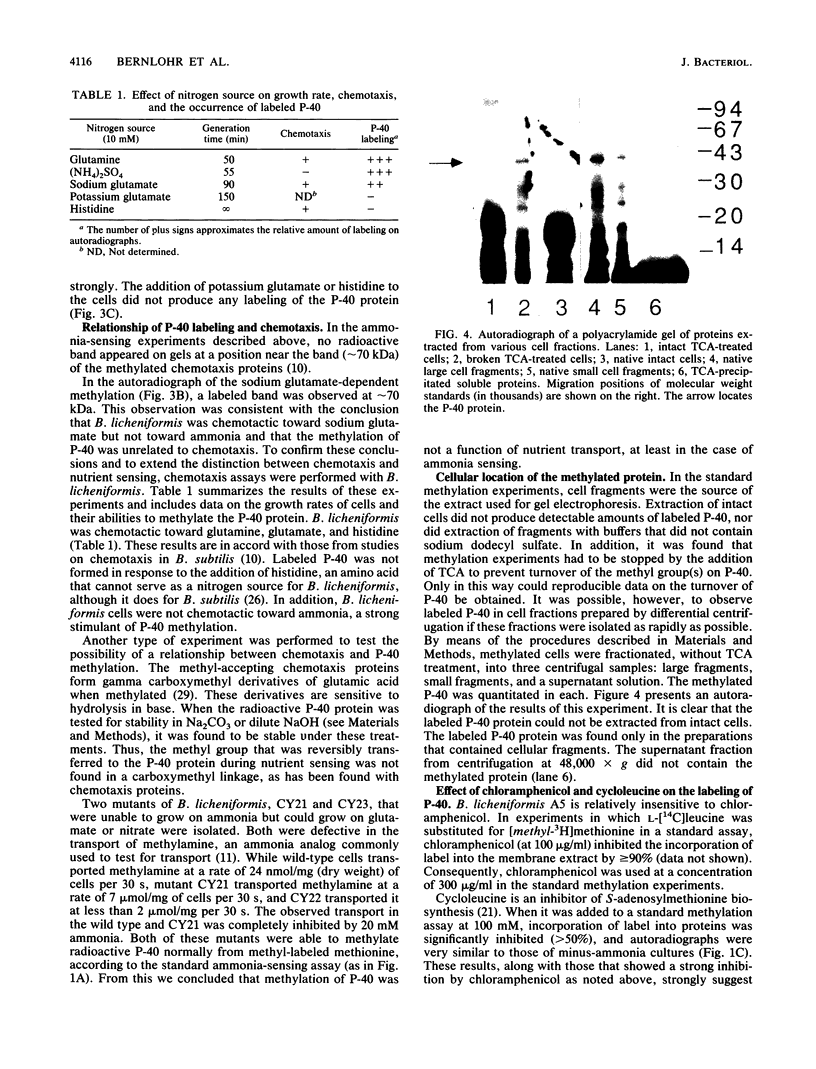
When nitrogen-starved vegetative cells of Bacillus licheniformis A5 were presented with a good nitrogen source in the presence of chloramphenicol and methyl-labeled methionine, a 40-kilodalton (kDa) protein was found to be reversibly methylated, with a half-life of approximately 10 to 15 min. The 40-kDa protein was strongly methylated in response to the addition of ammonia, glutamine, or sodium glutamate nitrogen sources that produce generation times of less than or equal to 90 min) but was very poorly methylated in the absence of a nitrogen source or in the presence of potassium glutamate or histidine (generation times of greater than 150 min). The methylated protein was found to be membrane associated, but the methylation reaction did not appear to be related to chemotaxis, because the spectrum of nutrients that promoted methylation was different from that which prompted a chemotactic response. In addition, the methyl residue on the 40-kDa protein was found to be alkali stable. Approximately 180 to 640 molecules of the methylated protein were found per cell. The characteristics of this methylated protein were consistent with the hypothesis that the reversible methylation of the protein functions in nutrient sensing to regulate growth, cell division, and the initiation of sporulation.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler J. A method for measuring chemotaxis and use of the method to determine optimum conditions for chemotaxis by Escherichia coli. J Gen Microbiol. 1973 Jan;74(1):77–91. doi: 10.1099/00221287-74-1-77. [DOI] [PubMed] [Google Scholar]
- Chelsky D., Gutterson N. I., Koshland D. E., Jr A diffusion assay for detection and quantitation of methyl-esterified proteins on polyacrylamide gels. Anal Biochem. 1984 Aug 15;141(1):143–148. doi: 10.1016/0003-2697(84)90437-8. [DOI] [PubMed] [Google Scholar]
- Clarke S., Mandelstam J. Dissociation of an early event in sporulation from chromosome replication in Bacillus subtilis. J Gen Microbiol. 1980 Dec;121(2):487–490. doi: 10.1099/00221287-121-2-487. [DOI] [PubMed] [Google Scholar]
- Donohue T. J., Bernlohr R. W. Properties of the Bacillus licheniformis A5 glutamine synthetase purified from cells grown in the presence of ammonia or nitrate. J Bacteriol. 1981 Aug;147(2):589–601. doi: 10.1128/jb.147.2.589-601.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubnau E. J., Cabane K., Smith I. Regulation of spo0H, an early sporulation gene in bacilli. J Bacteriol. 1987 Mar;169(3):1182–1191. doi: 10.1128/jb.169.3.1182-1191.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari F. A., Trach K., Hoch J. A. Sequence analysis of the spo0B locus reveals a polycistronic transcription unit. J Bacteriol. 1985 Feb;161(2):556–562. doi: 10.1128/jb.161.2.556-562.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields P. I., Schreier H. J., Saha A. L., Bernlohr R. W. Use of the beta-lactamase inhibitor clavulanic acid in the isolation of auxotrophic mutants of Bacillus licheniformis. J Bacteriol. 1984 Aug;159(2):803–804. doi: 10.1128/jb.159.2.803-804.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman D. J., Worobec S. W., Siegel R. B., Hecker R. V., Ordal G. W. Chemotaxis in Bacillus subtilis: effects of attractants on the level of methylation of methyl-accepting chemotaxis proteins and the role of demethylation in the adaptation process. Biochemistry. 1982 Mar 2;21(5):915–920. doi: 10.1021/bi00534a016. [DOI] [PubMed] [Google Scholar]
- Jayakumar A., Epstein W., Barnes E. M., Jr Characterization of ammonium (methylammonium)/potassium antiport in Escherichia coli. J Biol Chem. 1985 Jun 25;260(12):7528–7532. [PubMed] [Google Scholar]
- Kikkawa U., Nishizuka Y. The role of protein kinase C in transmembrane signalling. Annu Rev Cell Biol. 1986;2:149–178. doi: 10.1146/annurev.cb.02.110186.001053. [DOI] [PubMed] [Google Scholar]
- Komano T., Brown N., Inouye S., Inouye M. Phosphorylation and methylation of proteins during Myxococcus xanthus spore formation. J Bacteriol. 1982 Jul;151(1):114–118. doi: 10.1128/jb.151.1.114-118.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koshland D. E., Jr A response regulator model in a simple sensory system. Science. 1977 Jun 3;196(4294):1055–1063. doi: 10.1126/science.870969. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leung A., Rubinstein S., Yang C., Li J. W., Leighton T. Suppression of defective-sporulation phenotypes by mutations in the major sigma factor gene (rpoD) of Bacillus subtilis. Mol Gen Genet. 1985;201(1):96–98. doi: 10.1007/BF00397992. [DOI] [PubMed] [Google Scholar]
- Losick R., Pero J. Cascades of Sigma factors. Cell. 1981 Sep;25(3):582–584. doi: 10.1016/0092-8674(81)90164-1. [DOI] [PubMed] [Google Scholar]
- Macnab R. M., Aizawa S. Bacterial motility and the bacterial flagellar motor. Annu Rev Biophys Bioeng. 1984;13:51–83. doi: 10.1146/annurev.bb.13.060184.000411. [DOI] [PubMed] [Google Scholar]
- Niwano M., Taylor B. L. Novel sensory adaptation mechanism in bacterial chemotaxis to oxygen and phosphotransferase substrates. Proc Natl Acad Sci U S A. 1982 Jan;79(1):11–15. doi: 10.1073/pnas.79.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochi K., Freese E. A decrease in S-adenosylmethionine synthetase activity increases the probability of spontaneous sporulation. J Bacteriol. 1982 Oct;152(1):400–410. doi: 10.1128/jb.152.1.400-410.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ollington J. F., Haldenwang W. G., Huynh T. V., Losick R. Developmentally regulated transcription in a cloned segment of the Bacillus subtilis chromosome. J Bacteriol. 1981 Aug;147(2):432–442. doi: 10.1128/jb.147.2.432-442.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadaie Y., Kada T. Bacillus subtilis gene involved in cell division, sporulation, and exoenzyme secretion. J Bacteriol. 1985 Aug;163(2):648–653. doi: 10.1128/jb.163.2.648-653.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreier H. J., Smith T. M., Bernlohr R. W. Regulation of nitrogen catabolic enzymes in Bacillus spp. J Bacteriol. 1982 Aug;151(2):971–975. doi: 10.1128/jb.151.2.971-975.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel W. H., Donohue T., Bernlohr R. W. Determination of pools of tricarboxylic acid cycle and related acids in bacteria. Appl Environ Microbiol. 1977 Nov;34(5):512–517. doi: 10.1128/aem.34.5.512-517.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler B. Regulation of the assimilation of nitrogen compounds. Annu Rev Biochem. 1978;47:1127–1162. doi: 10.1146/annurev.bi.47.070178.005403. [DOI] [PubMed] [Google Scholar]
- Ullah A. H., Ordal G. W. In vivo and in vitro chemotactic methylation in Bacillus subtilis. J Bacteriol. 1981 Feb;145(2):958–965. doi: 10.1128/jb.145.2.958-965.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L. F., Doi R. H. Developmental expression of three proteins from the first gene of the RNA polymerase sigma 43 operon of Bacillus subtilis. J Bacteriol. 1987 Sep;169(9):4190–4195. doi: 10.1128/jb.169.9.4190-4195.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuber P., Losick R. Use of a lacZ fusion to study the role of the spoO genes of Bacillus subtilis in developmental regulation. Cell. 1983 Nov;35(1):275–283. doi: 10.1016/0092-8674(83)90230-1. [DOI] [PubMed] [Google Scholar]






