Abstract
Albright hereditary osteodystrophy (AHO), an autosomal dominant disorder characterized by short stature, obesity, and skeletal defects, is associated with heterozygous inactivating mutations of GNAS1, the gene encoding the heterotrimeric G protein α-subunit (Gsα) that couples multiple receptors to the stimulation of adenylyl cyclase. It has remained unclear why only some AHO patients present with multihormone resistance and why AHO patients demonstrate resistance to some hormones [e.g., parathyroid hormone (PTH)] but not to others (e.g., vasopressin), even though all activate adenylyl cyclase. We generated mice with a null allele of the mouse homolog Gnas. Homozygous Gs deficiency is embryonically lethal. Heterozygotes with maternal (m−/+) and paternal (+/p−) inheritance of the Gnas null allele have distinct phenotypes, suggesting that Gnas is an imprinted gene. PTH resistance is present in m−/+, but not +/p−, mice. Gsα expression in the renal cortex (the site of PTH action) is markedly reduced in m−/+ but not in +/p− mice, demonstrating that the Gnas paternal allele is imprinted in this tissue. Gnas is also imprinted in brown and white adipose tissue. The maximal physiological response to vasopressin (urinary concentrating ability) is normal in both m−/+ and +/p− mice and Gnas is not imprinted in the renal inner medulla (the site of vasopressin action). Tissue-specific imprinting of Gnas is likely the mechanism for variable and tissue-specific hormone resistance in these mice and a similar mechanism might explain the variable phenotype in AHO.
Heterotrimeric G proteins function as molecular switches in signal transduction pathways. Each G protein is composed of an α, β, and γ subunit, each the product of separate genes (1), and is defined by its α-subunit, which binds guanine nucleotides and interacts with specific receptors and effectors. The α-subunit of Gs (Gsα) is ubiquitously expressed and is known to couple multiple receptors to the stimulation of adenylyl cyclase and specific ion channels. However the physiological relevance of many Gs-coupled pathways remains to be defined and there are likely to be other Gs-coupled pathways that have yet to be identified. The human Gsα gene GNAS1 has been mapped to 20q13 (2) and appears to be single copy. Golf also stimulates adenylyl cyclase, but is only expressed in a small number of tissues. Heterozygous inactivating mutations of GNAS1 result in Albright hereditary osteodystrophy (AHO) (3), an autosomal dominant disorder characterized by short stature, obesity, brachydactyly, and subcutaneous ossifications. It is unclear why within a given kindred, some patients present with the somatic features of AHO alone [termed pseudopseudohypoparathyroidism (pseudoPHP)], while others present with AHO in association with multihormone resistance [termed pseudohypoparathyroidism type Ia (PHP Ia)], particularly because both PHP Ia and pseudoPHP patients have equivalent 50% deficiency of Gsα in some tissues and identical GNAS1 null mutations. It is also unclear why PHP Ia patients demonstrate resistance to some hormones [e.g., parathyroid hormone (PTH), thyrotropin] but not to others (e.g., vasopressin), even though all activate Gs-coupled pathways. It has been suggested that variable and tissue-specific hormone resistance in AHO might be due to variations in the expression of other signaling components (4).
The requirement for both maternal and paternal genetic contributions for normal mammalian development was the first evidence that a small number of genes are subject to genomic imprinting, an epigenetic phenomenon by which one allele (paternal or maternal) has partial or total loss of expression (5). Uniparental disomies (UPD) that result in abnormal development identified specific chromosome regions containing imprinted genes (6). The mouse Gsα gene (Gnas) has been mapped within a 20 centimorgan region between breakpoints T2Wa and T28H on distal chromosome 2 that is syntenic to human 20q13 and is presumed to contain one or more imprinted genes (7, 8). Fourteen genes within this region showed no evidence for imprinting (9, 10) and to date no imprinted genes have been identified within this region. A retrospective clinical review of AHO patients suggested that GNAS1 might be a paternally imprinted gene (11). However, one study failed to demonstrate imprinting of GNAS1 in human fetal tissues (12) and another in mice described imprinting in a manner opposite to that predicted by observations in AHO (13). Also, a case describing paternal transmission of PHP Ia was not in support of imprinting of GNAS1 (14). It still remains unclear whether or not the human or mouse Gsα genes are in fact imprinted.
In this paper we present an initial characterization of mice in which Gnas was disrupted by targeted mutagenesis and demonstrate that Gnas is imprinted in a tissue-specific manner. Consistent with Gnas being an imprinted gene, heterozygotes with paternal (+/p−) and maternal (m−/+) transmission of the Gnas null allele (GsKO) have distinct phenotypes. M−/+, but not +/p−, mice, are resistant to PTH while both m−/+ and +/p− mice have a normal maximal physiological response to vasopressin. Gsα expression studies demonstrate that Gnas is imprinted with the paternal allele relatively inactive in renal cortex (the site of PTH action) and brown and white adipose tissue. In contrast, Gnas is not imprinted in the renal inner medulla (site of vasopressin action). Tissue-specific imprinting of Gnas is likely the mechanism for variable and tissue-specific hormone resistance in these mice, and likely explains the variable and tissue-specific hormone resistance in AHO. This model also demonstrates that Gs is an important regulator of multiple developmental, hormonal and metabolic pathways in vivo.
MATERIALS AND METHODS
Construction of Targeting Vector.
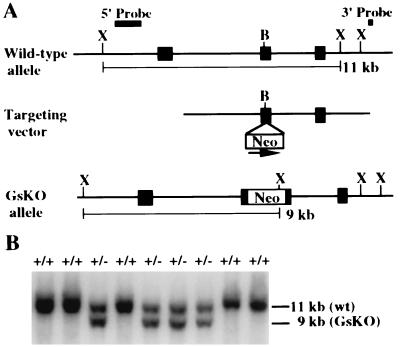
A 129/Sv genomic library (Stratagene) was screened with a 48-base oligonucleotide containing the coding sequence of Gnas exon 3 and a clone containing a 12-kb insert including Gnas exons 1–3 was isolated. A SalI-XhoI cassette containing the neomycin resistance gene downstream of the glucokinase promoter (GK-Neo) transcriptional unit (15) was ligated into a BamHI site in Gnas exon 2 in the same orientation by partial filling and ligation. The mutagenized clone was digested with SmaI and SstI and purified by agarose gel electrophoresis to generate a targeting vector with a left arm of 3.6 kb of homology and right arm of 4.9 kb of homology (Fig. 1A).
Figure 1.
Generation of Gnas knockout (GsKO) mice. (A) Schematic diagram of the wild type Gnas allele (Top) with exons 1–3 depicted as black boxes. The targeting vector (Middle) was generated by insertion of the GK-Neo cassette into a BamHI (B) site within exon 2 in the same orientation as Gnas (arrow), followed by digestion with SmaI and SstI (the SstI site is contributed by the vector). The recombinant GsKO allele is shown below. The position of the 5′ probe, a NotI-SmaI fragment, is shown above. After digestion with XmnI (X), this probe is predicted to hybridize to an 11-kb genomic fragment in the wild type allele and a 9-kb genomic fragment in the GsKO allele. The position of the 3′ probe used to confirm correct insertion of the targeting vector at the 3′ end is shown above. Southern hybridization using this probe is not shown. (B) Southern hybridization of F1 progeny derived from mating of a GsKO chimera with a CD1 mouse. Genomic DNA was digested with XmnI and hybridized with the 5′ probe. The genotype of individual animals is indicated above each lane.
Gene Targeting and Generation of Knockout Mice.
J-1 embryonic stem cells (kind gift of R. Jaenisch) were maintained, electroporated, and G418-selected as described (16). Each clone was analyzed by Southern hybridization after digestion with XmnI using a genomic fragment upstream of the targeting vector (Fig. 1A). Single insertion of the targeting vector was confirmed by Southern hybridization with several enzymes by using GK-Neo as a probe, and correct insertion at the 3′ end was confirmed by Southern hybridization using a 50-base oligonucleotide downstream of the targeting vector as probe (data not shown, Fig. 1A). Microinjection of embryonic stem cells and transfer of 3.5 days postcoitus embryos was performed as described (17). Chimeric males were mated with C57BL6, 129/Sv and CD1 (Charles River Breeding Laboratories) females and germ line transmission was achieved with CD1 matings. Genotyping of offspring of subsequent heterozygote × heterozygote crosses was performed by Southern hybridization. Genotyping of offspring of wild type × heterozygote crosses was performed by Southern hybridization or duplex PCR (18) by using primers for the β-globin gene promoter (5′-CCACAGATCCTATTGCCATGCCC-3′ and 5′-CATAAGACCCCAGTAATGAGCC-3′, 0.3 μM each) (19), which amplify a 501-bp product, and Neo-specific primers (5′-GATCGGCCATTGAACAAGATG-3′ and 5′-AAGGTGAGATGACAGGAGATC-3′, 0.5 μM each), which amplify a 310-bp product. For transplantation experiments fertilized eggs were removed from donor mothers and transplanted into foster mothers (20).
Blood and Tissue Analysis.
Ionized calcium was measured on an AVL 988–4 analyzer and corrected for pH. Serum phosphorus was determined by Ani-Lytics (Gaithersburg, MD). Serum PTH was measured by a rat PTH immunoradiometric assay (Nichols Institute, San Juan Capistrano, CA). For histology tissues were fixed in 10% neutral buffered formalin (4% formaldehyde), paraffin-embedded and sections were stained with hematoxylin and eosin.
Membrane Preparations and Immunoblotting.
Gonadal fat pads from 2–3-month-old male mice were minced and digested with collagenase for 30 min as described (21) and adipocytes were then washed three times in Krebs-Ringer medium (pH 7.4) containing 10 mM NaHCO3, 30 mM Hepes, and 200 nM adenosine without albumin. Total membranes were prepared as described (22). Kidneys were dissected and tissue fragments homogenized as described (23) and total membranes isolated with a 200,000 × g centrifugation. Protein concentrations were determined using the BCA kit (Pierce). Samples were electrophoresed in 10% Tricine-SDS gels (NOVEX, San Diego) and transferred to polyvinylidene difluoride filters (Immobilon-P, Millipore) by electroblotting. Filters were blocked in Tris-buffered saline with 0.5% Tween-20 (TBST) and 1% BSA at 25°C for 1 h, then incubated overnight at 25°C with affinity-purified RM antibody (2 μg/ml), directed to the C-terminal decapeptide of Gsα (24), in TBST, 1% BSA. After three washes in TBST, filters were incubated for 2 h in TBST, 1% BSA with 125I-protein A (NEN/DuPont), 0.1 μCi/ml (1 Ci = 37 GBq), and then rewashed three times with TBST. Bands were quantified with a PhosphorImager (Fuji BAS1000).
RNA Isolation and Northern Hybridization.
Total RNA was isolated from tissues by using Rneasy Mini kit (Qiagen, Chatsworth, CA), separated on 0.8% agarose, 6.66% formaldehyde gels and then transferred onto nylon membranes (Immobilon-N, Millipore). Membranes were hybridized with a 32P-labeled 1.2-kb EcoRI fragment or a 0.27-kb BglII-NsiI fragment of rat Gsα cDNA (25) in 5× standard saline phosphate/EDTA (0.18 M NaCl/10 mM phosphate, pH 7.4/1 mM EDTA), 10× Denhardt’s solution, 1% SDS, 50% formamide, and denatured salmon sperm DNA (0.5 mg/ml) at 50°C overnight. Membranes were then washed in 1 × standard saline phosphate/EDTA, 0.2% SDS at room temperature for 5 min and then twice at 50°C for 25 min.
In Situ Hybridization.
In situ hybridization was performed on frozen sections of renal cortex from adult mice as described (26) using 35S-labeled antisense and sense Gnas riboprobes (spanning residues 1058–1374 in ref. 27).
Determination of cAMP Generation in Renal Proximal Tubules.
Renal cortices were dissected and proximal tubule suspensions (≈95% enriched) were prepared by collagenase digestion and isolation on a Percoll gradient as described (28). The PTH analog [Nle8,18, Tyr34]bovine PTH(1–34) [abbreviated bPTH(1–34)] was a generous gift of E. Schipani (Massachusetts General Hospital, Boston). Suspensions were incubated in DMEM-F12 medium (pH 7.4), containing 4 mM glycine, 1 mM heptanoic acid, 0.5 mM 3-isobutyl-1-methylxanthine (IBMX) (all from Sigma), and various concentrations of bPTH(1–34) for 5 min at 37°C. Incubations were terminated with an equal volume of ice-cold 10% trichloroacetic acid followed by centrifugation at 2,300 × g for 10 min. The pellet was solubilized in 0.5% Triton X-100/0.25 M NaOH, and protein was determined by using a dye-binding assay (Bio-Rad). The supernatant was extracted with water-saturated ether four times and then dried in a Speed-Vac. The amount of cAMP in the dried pellets was determined by ELISA (Cayman Chemical, Ann Arbor MI). Incubations were performed in duplicate.
RESULTS
Generation of GsKO Mice.
GsKO mice were generated by targeted mutagenesis. A targeting vector was made with insertion of a GK-Neo cassette (15) into Gnas exon 2 (Fig. 1A). This region of the mouse and human genes encoding Gsα are structurally similar (29). Germline transmission of the GsKO allele was achieved by mating male chimeras with CD1 females. Correct targeting was confirmed by Southern hybridization using an upstream genomic fragment (Fig. 1B), GK-Neo and an oligonucleotide downstream of the targeting vector as probes (data not shown).
Homozygous Disruption of Gnas is Embryonically Lethal.
Mating of heterozygous GsKO mice results in no homozygous GsKO mice (of 83 genotyped) at birth, indicating that homozygous Gnas disruption is embryonically lethal, as one might predict for a non-redundant, ubiquitously expressed gene whose product is critical for many signaling pathways. This is further emphasized by the fact that even heterozygous disruption of Gnas is associated with significant early postnatal lethality (see below). Preliminary evaluation of embryos suggests that homozygous GsKO embryos are able to undergo implantation but stop developing normally before day E10.5.
Maternal vs. Paternal Inheritance of the GsKO Allele Leads to Distinct Phenotypes.
Female heterozygotes were mated to normal males and normal females were mated with male heterozygotes to generate m−/+ and +/p− offspring, respectively. Both m−/+ (47.5% of 122 newborns) and +/p− (50.4% of 135 newborns) offspring are born with the expected Mendelian frequency. M−/+ newborns have wide, square shaped bodies, subcutaneous edema (which spontaneously resolves within 3 days) and higher birth weights (m−/+ 1.85 ± 0.04 g (n = 32) v. maternal +/+ 1.62 ± 0.03 g (n = 40); P < 0.05, Student’s t test; Fig. 2). After 6–21 days, 80% of these mice develop ataxia, tremor, imbalance, a poor righting reflex, and difficulty breathing and die. Measurements of serum glucose (mean 44 ± 15 mg/dl, n = 17, range 8–255) and calcium (mean 10.33 ± 0.38 mg/dl, n = 12, range 8.16–12.6) rule out hypoglycemia or hypocalcemia as the cause for the neurological manifestations. These mice, but not normal or +/p− mice of the same age, have delayed development of the cerebellar cortex, with persistence of superficial granular cells. Detailed histological surveys of these mice are also notable for thymic cortical atrophy and immaturity of the kidneys. In contrast to m−/+ newborns, +/p− newborns have narrow bodies, arched backs, and lower birth weights (+/p− 1.37 ± 0.04 g (n = 15) vs. paternal +/+ 1.75 ± 0.05 g (n = 15); P < 0.05, Student’s t test), the majority of which (77%) do not suckle milk, become inactive, and die within 24 h after birth (Fig. 2).
Figure 2.
M−/+ and +/p− neonates have distinct physical characterisitics. Shown are (a) +/p−, (b) m−/+ neonates above and (c, d) normal littermates below. Note lack of milk in stomach of +/p− pup.
To rule out the possibility that differences in uterine environment or maternal nurturing contribute to the distinct phenotypes of +/p− and m−/+ mice, transplantation experiments were performed. Transplantation of fertilized eggs from heterozygous to normal mothers or from normal to heterozygous mothers had no effect on the observed phenotype or on the timing or extent of lethality of mutant offspring, confirming that the phenotype is determined by the parental transmission of the GsKO allele and not by maternal effects. It is interesting to note that the early phenotypes that we observed in m−/+ and +/p− mice are strikingly similar to those previously described for mice with paternal UPD/maternal deletion and maternal UPD/paternal deletion of the distal chromosome 2 imprinted region, respectively (7, 30, 31). These observations strongly suggest that Gnas is imprinted and is probably one of the genes that contributes to the UPD phenotypes.
While longer periods of survival of mice with UPDs of the imprinted region was not described previously, we observed that a significant minority of GsKO heterozygotes (both m−/+ and +/p−) do survive beyond the first several weeks. This difference in survival between GsKO and UPD mice may be due to differences in genetic background (30, 31). m−/+ and +/p− mice continued to show other phenotypic differences after birth, including differences in the dorsal neck (convex with several large skin folds in m−/+, concave in +/p− mice) and ears (round and inset in maternal ±, normal in +/p− mice). By day 16 these differences were not noticeable. Delayed growth and development were evident in both +/p− and m−/+ mice. With few exceptions, both +/p− and m−/+ mice that survive to weaning (3 weeks) are fertile and have a normal life span up to 27 months.
m−/+ but Not +/p− Mice Are Resistant to PTH.
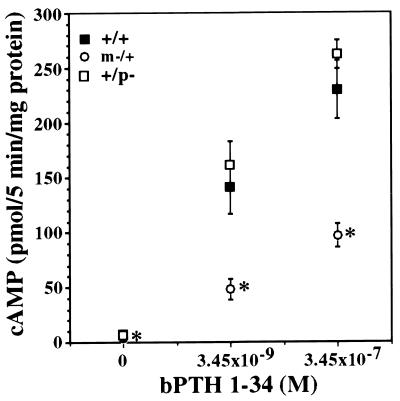
PTH resistance is characterized by decreased serum calcium and elevated serum phosphorus and PTH. These findings are present in adult m−/+ but not +/p− mice (Table 1). Consistent with these results, the ability of a PTH analog to stimulate cAMP production in isolated renal proximal tubules (the primary site of PTH action in the kidney) is significantly reduced in m−/+ mice but not in +/p− mice (Fig. 3). This is analogous to what is observed in humans, with maternal but not paternal transmission of AHO leading to PTH resistance (11) and markedly decreased urinary cAMP in response to PTH (32). The hypocalcemia and PTH elevations in m−/+ mice were modest. Histological examination of bones from several sites and measurements of serum alkaline phosphatase showed no overt evidence of increased bone turnover or osteitis fibrosa cystica, which are associated with elevated PTH.
Table 1.
Calcium and phosphorous metabolism in GsKO heterozygotes
| Mice | Ionized calcium, mM | Serum phosphorus, mg/dl | PTH, pg/ml |
|---|---|---|---|
| +/+ | 1.26 ± 0.02 (16) | 10.9 ± 0.8 (8) | 42 ± 9 (16) |
| m−/+ | 1.19 ± 0.01 (12)* | 13.5 ± 0.8 (5) | 95 ± 21 (7)* |
| +/p− | 1.23 ± 0.02 (9) | 9.2 ± 0.4 (4) | 36 ± 8 (10) |
Results are expressed as mean ± SEM (n) and are derived from male and female adult animals >2.5 months of age. Sex had no significant effect on these parameters.
P ≤ 0.05 by one-factor ANOVA.
Figure 3.
bPTH(1–34)-stimulated cAMP production in isolated renal proximal tubules. Proximal tubule suspensions were incubated with the indicated concentrations of bPTH(1–34) in the presence of 0.5 mM IBMX. Maximal cAMP response was achieved in normal mice at a concentration of 3.45 × 10−8. Data are the mean ± SEM of 3 animals for +/p− and m−/+ mice and 6 animals for +/+ mice (except at the highest bPTH concentration in which the data are from 5 animals). Asterisks indicate that the response was significantly reduced in m−/+ mice at all concentrations of agonist.
Imprinting of the Gnas Paternal Allele in Renal Cortex.
The presence of PTH resistance in m−/+ but not +/p− mice suggests that the imprinting model might be relevant to GsKO mice. If Gnas is paternally imprinted, maternal transmission of GsKO should result in little or no Gsα expression (due to imprinting of the paternal allele and mutation of the maternal allele) while paternal transmission should result in Gsα expression that is similar to that in normal mice (because the mutation is on the imprinted allele). We examined Gsα expression by immunoblot in membranes from the renal cortex, which is enriched in proximal tubules (the major renal site of PTH action) and glomeruli but also contains portions of the distal tubule. Consistent with the imprinting model, Gsα protein expression was significantly reduced in m−/+ but not in +/p− mice (m−/+ 36 ± 8% of normal vs. +/p− 95 ± 7% of normal, four pairs per group, P < 0.05 for +/p− vs. m−/+ by Student’s t test, Fig. 4A). Examination of Gnas mRNA expression in isolated proximal tubules by Northern hybridization gave similar results (m−/+ 38 ± 4% of normal vs. +/p− 89 ± 9% of normal, three pairs per group, P < 0.05, Student’s t test). We then examined Gnas mRNA expression in glomeruli and proximal tubules by in situ hybridization on sections of renal cortex from adult mice. Hybridization with an antisense Gnas riboprobe showed that Gnas expression in the glomeruli of +/p− and m−/+ mice was similar and somewhat less than that observed in +/+ mice, consistent with lack of imprinting of Gnas in glomeruli. In contrast Gnas expression in the surrounding proximal tubules was similar to control subjects in +/p− mice and reduced in m−/+ mice (Fig. 5 A vs. B), confirming that Gnas paternal allele is imprinted in the proximal tubule.
Figure 4.
Gsα expression in renal cortex and inner medulla and white and brown adipose tissue. In A–C the results of paired littermates (normal +/+, mutant +/−, or −/+) are shown. (A) Immunoblots of renal cortical membranes (above, CTX, 8 μg protein/lane) and renal inner medulla membranes (below, IM, 5 μg protein/lane) incubated with RM antibody followed by 125I-protein A. (B) Immunoblots of membranes prepared from adipocytes isolated from gonadal fat pads (3 μg protein/lane) incubated with RM antibody. (C) Northern hybridization of total RNA isolated from intrascapular brown adipose tissue (4 μg/lane) with a 32P-labeled Gsα rat cDNA probe.
Figure 5.
In situ hybridization of renal cortex from adult mice. Bright field microscopic images of renal cortex from (A) +/p−, (B) m−/+, and (C) +/+ mice after hybridization with antisense Gnas riboprobe and (D) +/+ mouse after hybridization with sense Gnas riboprobe. Glomeruli are indicated with arrows. (×400.)
Gnas Is Not Imprinted in the Renal Inner Medulla.
PHP Ia patients have resistance to some hormones (e.g., PTH, thyrotropin) but not to others (e.g., vasopressin, adrenocorticotropin), even though all activate Gs-coupled pathways. Tissues in which the gene is not imprinted would be expected to have a partial 50% decrease in Gsα expression regardless of parent-of-origin. A 50% decrease in Gsα expression would be expected to result in a moderately reduced cAMP response, as was observed for glucagon and isoproterenol in PHP Ia (33–35). This partial response is sufficient to produce a maximal physiological response (34, 36) and resistance to these hormones is not evident in PHP Ia. Therefore tissue-specific hormone resistance could be the result of tissue-specific imprinting of the Gsα gene.
To test this hypothesis in GsKO mice we determined the imprinting status of Gnas in a target tissue (renal medulla) for a hormone (vasopressin) for which resistance is not evident. As in PHP Ia patients (37), m−/+ mice are able to concentrate their urine normally in response to 48 h of water deprivation (m−/+ 3465 ± 96 mosm/kg vs. maternal +/+ 3518 ± 82 mosm/kg, five pairs of animals, P = 0.73 by Student’s t test), and therefore have a normal maximal physiological response to endogenous vasopressin. The expression of two proteins in more distal elements of the nephron that are regulated by vasopressin or cAMP (aquaporin 2 in collecting ducts and the Na-K transporter BSC1 in thick ascending limbs) were decreased by ≈50% in both m−/+ and +/p− mice (C. A. Ecelbarger and M. Knepper, unpublished data), suggesting that Gnas is not imprinted in the distal nephron. To confirm this we examined Gsα expression by both immunoblot and Northern hybridization in the renal inner medulla, which contains primarily collecting ducts (the target tissue for vasopressin). Parental transmission of the GsKO allele had no significant effect on Gsα protein or mRNA expression in heterozygotes relative to that in normal mice (protein: m−/+ 67 ± 6% of normal vs. +/p− 62 ± 9% of normal; four pairs per group; mRNA: m−/+ 52 ± 10% of normal vs. +/p− 62 ± 7% of normal; four pairs per group; Fig. 4A), consistent with lack of imprinting of Gnas in this tissue. Northern hybridization was also consistent with no imprinting of Gnas in lung and partial imprinting of the Gnas paternal allele in brain (data not shown).
Gnas Is Imprinted in Adipose Tissue.
Imprinting of the Gnas paternal allele was not limited to the renal proximal tubule. Gsα expression in brown and white adipose tissue was markedly decreased only in m−/+ mice (Fig. 4 B and C). M−/+ become obese in the early adult period (S.Y. and L.S.W., unpublished data) and obesity is a commonly associated with AHO. It is likely that decreased Gsα expression in adipose tissue leads to obesity in these mice because cAMP stimulates lipolysis and thermogenesis in adipose tissue. Surprisingly, +/p− mice were leaner than normal. The mechanism leading to decreased fat mass in +/p− mice is unclear but may not be relevant to humans because patients with paternal inheritance of AHO tend to also be obese.
DISCUSSION
Paternal UPD/maternal deletion and maternal UPD/paternal deletion of a region between breakpoints T2Wa and T28H on distal chromosome 2 result in distinct phenotypes and early lethality, strongly suggesting the existence of one or more imprinted genes within the region (7, 8). Evaluation of multiple genes within the region has failed to identify any that are imprinted (9, 10). We show that a null mutation in the Gnas maternal or paternal allele results in phenotypes strikingly similar to those of mice with paternal UPD/maternal deletion or maternal UPD/paternal deletion of the imprinted region, respectively. This strongly suggests that Gnas is an imprinted gene within this region. Imprinting of the Gnas paternal allele was confirmed by Gsα expression studies in various tissues of m−/+ and +/p− mice. Neuronatin (Nnat) has been shown to be an imprinted gene on distal chromosome 2 that maps outside of the T2Wa-T28H imprinted region (38). Given the large distance and the presence of multiple nonimprinted genes between Gnas and Nnat, it is likely that they lie within distinct imprinting domains.
It has remained unclear why heterozygous GNAS1 null mutations in man lead to multihormone resistance in some AHO patients (PHP Ia) but not in others (pseudoPHP). Analogous to observations in AHO patients (11), PTH resistance was present in m−/+ but not +/p− mice. Imprinting of the Gnas paternal allele should result in markedly reduced Gnas expression in m−/+ but not in +/p− mice, and this was observed in the renal proximal tubules and in adipose tissue. Reduced levels of Gsα protein in the proximal tubules of m−/+ but not +/p− mice is likely the explanation for the expression of PTH resistance only in m−/+ mice. This explanation is further supported by the observation that the ability of a PTH analog to stimulate cAMP production in proximal tubules was significantly reduced in m−/+ mice but not in +/p− mice, analogous to the urinary cAMP response to PTH in PHP Ia and pseudoPHP, respectively (32).
While significantly reduced, there is still a cAMP response to bPTH(1–34) in the proximal tubules of m−/+ mice. This may indicate that imprinting of the Gnas paternal allele in proximal tubule is only partial or restricted to specific segments of the proximal tubule. Results of in situ hybridization and Northern hybridization show that Gnas is expressed to some degree in the proximal tubules of m−/+ mice. It is also possible that PTH can stimulate a calcium-sensitive adenylyl cyclase through a Gq/11-coupled pathway or that contamination of the proximal tubule preparations with glomeruli and distal nephron segments contributes to this residual activity. The presence of a cAMP response to PTH in proximal tubules might explain why PTH resistance in m−/+ mice is relatively mild, with borderline hypocalcemia and modest elevation of serum PTH.
Williamson et al. (13) concluded that the Gnas maternal, rather than paternal, allele is imprinted in renal glomeruli based on in situ hybridization experiments that showed that Gnas expression in glomeruli was increased in mice with paternal UPD/maternal deletion and decreased in those with maternal UPD/paternal deletion of distal chromosome 2. Imprinting of the Gnas maternal allele should lead to markedly decreased Gnas expression in +/p− but not m−/+ mice. Our in situ hybridization experiments on adult GsKO heterozygotes were consistent with lack of imprinting of Gnas in glomeruli. The differences in Gnas expression observed by Williamson may reflect differences in the maturation state of glomeruli in their UPD mice because they studied animals during the period of glomerular maturation and we observe that glomerular maturation in early postnatal m−/+ and +/p− mice occur at different rates. Their results may also be secondary to perturbations in other genes located within the UPD region.
It has also remained unclear why PHP Ia patients are resistant to some hormones (e.g., PTH) but not to others (e.g., vasopressin) that activate Gs-coupled pathways. We hypothesized that if Gnas is imprinted in a tissue-specific manner (as has been demonstrated for other imprinted genes, refs. 39–42) then hormone resistance would only be evident in tissues in which the gene is imprinted. Tissues in which the gene is not imprinted would be expected to have a partial 50% decrease in Gsα expression regardless of parent-of-origin. A 50% decrease in Gsα expression would be expected to result in a moderately reduced cAMP response, as was observed for glucagon and isoproterenol in PHP Ia (33–35). This partial response is sufficient to produce a maximal physiological response (34, 36) and therefore resistance to these hormones is not evident. The lack of imprinting in the inner medulla is likely the reason that these mice, like PHP Ia patients, maintain a normal maximal physiological response to vasopressin. In PHP Ia patients, PTH actions on the proximal tubule (increased 1, 25 dihydroxyvitamin D synthesis, decreased phosphate reabsorption) are diminished while PTH actions on distal portions of the nephron (increased calcium reabsorption) are maintained (43). Imprinting of GNAS1 in the proximal tubule but not in more distal portions of the nephron could explain this clinical observation. Because all portions of the nephron except the collecting duct are derived from the metanephric blastema, differences in embryonic origin alone cannot explain the differences in imprinting status of Gnas within different portions of the nephron.
The GsKO model provides compelling evidence that the variable and tissue-specific hormone resistance observed in the human disease AHO results from tissue-specific imprinting of the GNAS1 gene. Although most or all human homologs of genes that are imprinted in mice have been shown to be imprinted, further studies are required to prove that the human GNAS1 gene is imprinted. McCune-Albright syndrome, a sporadic disease characterized by multiple hyperfunctioning endocrine glands, fibrous dysplasia of bone, and segmental skin hyperpigmentation, is associated with heterozygous activating mutations of GNAS1 in a mosaic distribution (44). If GNAS1 is imprinted, then the presentation of individuals with McCune-Albright syndrome may be dependent not only on the tissue distribution of the mutation but also on which allele bears the mutation.
The obesity observed in m−/+ mice (and humans with AHO) is likely the consequence of markedly reduced Gsα expression in adipose tissue resulting from imprinting of Gnas. Surprisingly, +/p− mice also had an abnormal phenotype (early lethality and decreased fat mass). The GsKO model is the first example of two distinct abnormal phenotypes resulting from disruption of a single imprinted genetic locus. However the mechanisms underlying the +/p− phenotype is at present not obvious. The GsKO mouse demonstrates that Gs plays a major role in developmental processes and lipid metabolism. Preliminary observations in these mice also suggest that Gs is a negative regulator of insulin signaling (S.Y. and L.S.W., unpublished data) and may have important roles in the regulation of immune cells and renal function. This animal model will be most useful in further understanding the many physiological roles of Gs in vivo and the phenomenon of genomic imprinting.
Acknowledgments
We thank C. A. Ecelbarger, M. Knepper, C. Mitchell, R. B. Doctor, H. Chen, M. Quon, Z. Liu, J. Zhou, S. Cecco, C. Deng, S. Huang, K. Takeda, P. Goldsmith, R. Vinitsky, S. Pellegrino, and C. Woodard for technical assistance and advice; E. Schipani for providing bovine PTH (1–34) analog; and A. Spiegel and M. Reitman for critical reading of the manuscript.
ABBREVIATIONS
- AHO
Albright hereditary osteodystrophy
- Gsα
α-subunit of heterotrimeric G protein Gs
- GsKO
Gnas allele with targeted null mutation
- PTH
parathyroid hormone
- PHP Ia
pseudohypoparathyroidism type Ia
- IBMX
3-isobutyl-1-methyxanthine
- UPD
uniparental disomy
- bPTH(1–34)
[Nle8,18, Tyr34]bovine PTH(1–34)
- m−/+
heterozygote with maternal inheritance of the GsKO allele
- +/p−
heterozygote with paternal inheritance of the GsKO allele
- GK-Neo
neomycin resistance gene downstream of the glucokinase promoter
- TBST
Tris-buffered saline with 0.5% Tween-20
References
- 1.Spiegel A M, Shenker A, Weinstein L S. Endocr Rev. 1992;13:536–565. doi: 10.1210/edrv-13-3-536. [DOI] [PubMed] [Google Scholar]
- 2.Gejman P V, Weinstein L S, Martinez M, Spiegel A M, Cao Q, Hsieh W-T, Hoehe M R, Gershon E S. Genomics. 1991;9:782–783. doi: 10.1016/0888-7543(91)90377-q. [DOI] [PubMed] [Google Scholar]
- 3.Weinstein L S. In: G Proteins, Receptors, and Disease. Spiegel A M, editor. Totowa, NJ: Humana; 1998. pp. 23–56. [Google Scholar]
- 4.Spiegel A M. N Engl J Med. 1990;322:1461–1462. doi: 10.1056/NEJM199005173222010. [DOI] [PubMed] [Google Scholar]
- 5.Bartolomei M S, Tilghman S M. Annu Rev Genet. 1997;31:493–525. doi: 10.1146/annurev.genet.31.1.493. [DOI] [PubMed] [Google Scholar]
- 6.Beechey C V, Cattanach B M, Searle A G. Mouse Genome. 1990;87:64–65. [Google Scholar]
- 7.Cattanach B M, Kirk M. Nature (London) 1985;315:496–498. doi: 10.1038/315496a0. [DOI] [PubMed] [Google Scholar]
- 8.Peters J, Beechey C V, Ball S T, Evans E P. Genet Res. 1994;63:169–174. doi: 10.1017/s0016672300032316. [DOI] [PubMed] [Google Scholar]
- 9.Williamson C M, Dutton E R, Abbott C M, Beechey C V, Ball S T, Peters J. Genet Res. 1995;65:83–93. doi: 10.1017/s0016672300033103. [DOI] [PubMed] [Google Scholar]
- 10.Marker P C, King J A, Copeland N A, Jenkins N A, Kingsley D M. Genomics. 1995;28:576–580. doi: 10.1006/geno.1995.1192. [DOI] [PubMed] [Google Scholar]
- 11.Davies S J, Hughes H E. J Med Genet. 1993;30:101–103. doi: 10.1136/jmg.30.2.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Campbell R, Gosden C M, Bonthron D T. J Med Genet. 1994;31:607–614. doi: 10.1136/jmg.31.8.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Williamson C M, Schofield J, Dutton E R, Seymour A, Beechey C V, Edwards Y H, Peters J. Genomics. 1996;36:280–287. doi: 10.1006/geno.1996.0463. [DOI] [PubMed] [Google Scholar]
- 14.Schuster V, Kress W, Kruse K. J Med Genet. 1994;31:84. doi: 10.1136/jmg.31.1.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Soriano P, Montgomery C, Geske R, Bradley A. Cell. 1991;64:693–702. doi: 10.1016/0092-8674(91)90499-o. [DOI] [PubMed] [Google Scholar]
- 16.Accili D, Drago J, Lee E J, Johnson M D, Cool M H, Salvatore P, Asico L D, José P A, Taylor S I, Westphal H. Nat Genet. 1996;12:106–109. doi: 10.1038/ng0196-106. [DOI] [PubMed] [Google Scholar]
- 17.Drago J, Gerfen C R, Lachowicz J E, Steiner H, Hollon T R, Love P E, Ooi G T, Grinberg A, Lee E J, Huang S P. Proc Natl Acad Sci USA. 1994;91:12564–12568. doi: 10.1073/pnas.91.26.12564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weinstein L S, Gejman P V, Friedman E, Kadowaki T, Collins R M, Gershon E S, Spiegel A M. Proc Natl Acad Sci USA. 1990;87:8287–8290. doi: 10.1073/pnas.87.21.8287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reitman M, Lee E, Westphal H, Felsenfeld G. Mol Cell Biol. 1993;13:3990–3998. doi: 10.1128/mcb.13.7.3990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brinster H L, Chen N Y, Trumbauer M E, Yagle M K, Palmiter R D. Proc Natl Acad Sci USA. 1985;82:4438–4442. doi: 10.1073/pnas.82.13.4438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Quon M J, Zarnowski M J, Guerre-Millo M, Sierra M, Taylor S I, Cushman S W. Biochem Biophys Res Commun. 1993;194:338–346. doi: 10.1006/bbrc.1993.1825. [DOI] [PubMed] [Google Scholar]
- 22.Quon M J, Chen H, Ing B L, Liu M-L, Zarnowski M J, Yonezawa K, Kasuga M, Cushman S W, Taylor S I. Mol Cell Biol. 1995;15:5403–5411. doi: 10.1128/mcb.15.10.5403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ecelbarger C A, Terris J, Frindt G, Echevarria M, Marples D, Nielson S, Knepper M A. Am J Physiol. 1995;269:F663–F672. doi: 10.1152/ajprenal.1995.269.5.F663. [DOI] [PubMed] [Google Scholar]
- 24.Simonds W F, Goldsmith P K, Woodard C J, Unson C G, Spiegel A M. FEBS Lett. 1989;249:189–194. doi: 10.1016/0014-5793(89)80622-2. [DOI] [PubMed] [Google Scholar]
- 25.Jones D T, Reed R R. J Biol Chem. 1987;262:14241–14249. [PubMed] [Google Scholar]
- 26.Bondy C A, Zhou J, Lee W H. In: Handbook of Endocrine Research Techniques. de Pablo F, Scanes C G, editors. New York: Academic; 1993. pp. 266–288. [Google Scholar]
- 27.Rall T, Harris B A. FEBS Lett. 1987;224:365–371. doi: 10.1016/0014-5793(87)80486-6. [DOI] [PubMed] [Google Scholar]
- 28.Doctor R B, Chen J, Peters L L, Lux S E, Mandel L J. Am J Physiol. 1998;274:F129–F138. doi: 10.1152/ajprenal.1998.274.1.F129. [DOI] [PubMed] [Google Scholar]
- 29.Kozasa T, Itoh H, Tsukamoto T, Kaziro Y. Proc Natl Acad Sci USA. 1988;85:2081–2085. doi: 10.1073/pnas.85.7.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cattanach, B. M. (1986) J. Embryol. Exp. Morph. 97 Suppl., 137–150. [PubMed]
- 31.Cattanach, B. M. & Beechey, C. V. (1990) Development (Cambridge, U.K.) Suppl., 63–72. [PubMed]
- 32.Levine M A, Jap T S, Mauseth R S, Downs R W, Spiegel A M. J Clin Endocrinol Metab. 1986;62:497–502. doi: 10.1210/jcem-62-3-497. [DOI] [PubMed] [Google Scholar]
- 33.Levine M A, Downs R W, Jr, Moses A M, Breslau N A, Marx S J, Lasker R D, Rizzoli R E, Aurbach G D, Spiegel A M. Am J Med. 1983;74:545–556. doi: 10.1016/0002-9343(83)91008-2. [DOI] [PubMed] [Google Scholar]
- 34.Brickman A S, Carlson H E, Levin S R. J Clin Endocrinol Metab. 1986;63:1354–1360. doi: 10.1210/jcem-63-6-1354. [DOI] [PubMed] [Google Scholar]
- 35.Carlson H E, Brickman A S. J Clin Endocrinol Metab. 1983;56:1323–1326. doi: 10.1210/jcem-56-6-1323. [DOI] [PubMed] [Google Scholar]
- 36.Carlson H E, Brickman A S, Burns T W, Langley P E. J Clin Endocrinol Metab. 1985;61:382–384. doi: 10.1210/jcem-61-2-382. [DOI] [PubMed] [Google Scholar]
- 37.Moses A M, Weinstock R S, Levine M A, Breslau N A. J Clin Endocrinol Metab. 1986;62:221–224. doi: 10.1210/jcem-62-1-221. [DOI] [PubMed] [Google Scholar]
- 38.Kikyo N, Williamson C M, John R M, Barton S C, Beechey C V, Ball S T, Cattanach B M, Surani M A, Peters J. Dev Biol. 1997;190:66–77. doi: 10.1006/dbio.1997.8681. [DOI] [PubMed] [Google Scholar]
- 39.Jinno Y, Yun K, Nishiwaki K, Kubota T, Ogawa O, Reeve A E, Niikawa N. Nat Genet. 1994;6:305–309. doi: 10.1038/ng0394-305. [DOI] [PubMed] [Google Scholar]
- 40.DeChiara T M, Robertson E J, Efstradiadis A. Cell. 1991;54:849–859. doi: 10.1016/0092-8674(91)90513-x. [DOI] [PubMed] [Google Scholar]
- 41.Giddings S J, King C D, Harman K W, Flood J F, Carnaghi L R. Nat Genet. 1994;6:310–313. doi: 10.1038/ng0394-310. [DOI] [PubMed] [Google Scholar]
- 42.Albrecht U, Sutcliffe J S, Cattanach B M, Beechey C, Armstrong D, Eichele G, Beaudet A L. Nat Genet. 1997;17:75–78. doi: 10.1038/ng0997-75. [DOI] [PubMed] [Google Scholar]
- 43.Stone M D, Hosking D J, Garcia-Himmelstine C, White D A, Rosenblum D, Worth H G. Bone. 1993;14:727–735. doi: 10.1016/8756-3282(93)90204-n. [DOI] [PubMed] [Google Scholar]
- 44.Weinstein L S, Shenker A, Gejman P V, Merino M J, Friedman E, Spiegel A M. N Engl J Med. 1991;325:1688–1695. doi: 10.1056/NEJM199112123252403. [DOI] [PubMed] [Google Scholar]