Abstract
Transformed cells can spontaneously silence genes by de novo methylation, and it is generally assumed that this is due to DNA methyltransferase activity. We have tested the alternative hypothesis that gene silencing could be due to the uptake of 5-methyl-dCMP into DNA, via the di- and triphosphonucleotides. 5-Methyl-dCMP would be present in cells from the ongoing repair of DNA. We have isolated a strain of Chinese hamster ovary (CHO) cells, designated HAM−, which spontaneously silences two tested genes at a very high frequency. We have shown that this strain incorporates 5-[3H]methyldeoxycytidine into 5-methylcytosine and thymine in DNA. It also has low 5-methyl-dCMP deaminase activity. Another HAM+ strain has high deaminase activity and a very low frequency of gene silencing. The starting strain, CHO K1, has a phenotype intermediate between HAM− and HAM+.
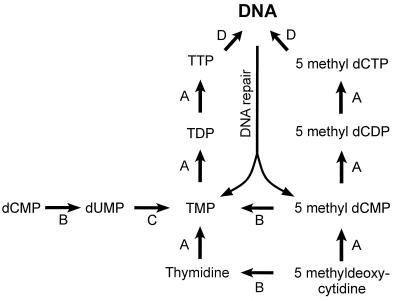
In normal diploid somatic cells, the distribution of 5-methylcytosine (5-methyl-C) is tightly controlled, whereas this control is lost in transformed neoplastic cells (1–4). In these transformed cells, cytosine residues can become methylated by a process generally referred to as de novo methylation, and this can lead to a loss of gene expression, or gene silencing (5–7). DNA is subject to continual spontaneous damage, for example, from the loss of purine or pyrimidine bases (abasic sites) or the deamination of cytosine to uracil (8). Some of the ongoing repair processes lead to DNA turnover with the formation of nucleotide monophosphates, including 5-methyldeoxycytidine monophosphate (5-methyl-dCMP). In normal cells, it is essential that this is not re-incorporated into DNA in random positions, as it would drastically alter the normal distribution of 5-methyl-C in DNA and might have aberrant effects on gene expression. There are two ways in which this can be prevented. The first is through the enzymic deamination of 5-methyl-dCMP to thymidine monophosphate (TMP), which can then be incorporated into DNA (see Fig. 1). The second is the reported inactivity of dCMP kinase, or any other kinase, on 5-methyl-dCMP (9–11). Thus, the diphosphonucleotide (5-methyl-dCDP) would not be produced. [It should be noted, however, that the kinase converting diphosphonucleotides to the triphosphates is not substrate specific (12, 13).]
Figure 1.
Pathways of uptake of pyrimidine nucleotides TMP and 5-methyldeoxycytidine monophosphate (5-methyl-dCMP) into DNA. A, kinases; D, DNA polymerase; B, deaminases; C, thymidylate synthetase. Aminopterin blocks the conversion of dUMP to TMP. Cells can grow in the presence of aminopterin if thymidine (and hypoxanthine) are provided exogenously (HAT medium). 5-Methyl-dC can substitute for thymidine if one or both deaminases are active (HAM medium). Strains resistant to BrdU, which lack thymidine kinase, can grow in HAM medium only if 5-methyl-dCMP deaminase is active.
It is well established that the regulation of gene activities in transformed cells is often abnormal, and this includes disruption of normal pyrimidine metabolism (14). The question therefore arises whether some de novo methylation could really be due to the conversion of 5-methyl-dCMP to 5-methyl-dCTP and the subsequent incorporation of the triphosphonucleotide directly to DNA. It is known that this can be achieved artificially in Chinese hamster ovary (CHO) cells, V79 cells, and normal human diploid fibroblasts, by permeabilizing the cells in the presence of 5-methyl-dCTP and showing that gene silencing can be induced (15–17). In this study, we have obtained evidence that 5-methyl-dCMP can be incorporated into DNA in a particular CHO cell strain that has a very high frequency of spontaneous gene silencing.
MATERIALS AND METHODS
Strains and Cell Culture.
All strains were derived from a subcloned population of CHO K1 cells. Cells were routinely grown in Eagle’s minimal essential medium (GIBCO/BRL) supplemented with nonessential amino acids, 10% fetal calf serum, penicillin, and streptomycin (0.06 mg/ml and 0.1 mg/ml, respectively), in 25-cm2 flasks, 5- or 10-cm plates, or in 24-well trays (1 ml/well). They were incubated at 37°C in 5% CO2. Cells were detached with trypsin and Versene (0.16% trypsin and 0.54 mM Versene), suspended in MEM, and counted with a Coulter Counter. Colony counts were made after 7–9 days of incubation and staining with Giemsa. Individual clones were isolated using cloning rings, transferred to wells and subsequently to flasks.
Selection Procedures.
Bromodeoxyuridine (BrdU)-resistant (BrdUR) colonies were selected in MEM containing 200 μg/ml BrdU. Reversions to BrdU sensitivity were selected in HAT medium (100 μM hypoxanthine, 0.4 μM aminopterin, and 16 μM thymidine by 1:50 dilution of a stock from Sigma). 6-Thioguanine (6-TG)-resistant (6-TGR) colonies were selected in MEM containing 2 μg/ml 6-TG. Reversions were selected in HAT medium. HAM medium contained 10% fetal calf serum, previously dialyzed to remove traces of thymidine, the same concentrations of aminopterin and hypoxanthine as HAT medium, and 5 μg/ml 5-methyldeoxycytidine (5-methyl-dC). The growth response to varying levels of 5-methyl-dC was measured by seeding 104 cells in wells and counting the cells 4 days later. Bromodeoxycytidine (BrdC) was used at 500 μg/ml or 1 mg/ml in MEM.
Treatment with 5-Azacytidine (5-aza-CR) and Ethyl Methanesulfonate (EMS).
6-TGR or BrdUR cells (106) were incubated for 24 h in a 25-cm2 flask. 1 μg/ml 5-aza-CR was added, and 24 h later the medium was changed. After 4–6 days of growth in MEM, 105 cells were tested for reactivation in HAT medium or transferred to flasks containing HAM medium (2.5 or 5 μg/ml 5-methyl-dC). BrdUS colonies were cloned from HAT medium. Mutagenesis was induced by incubating 105 cells for 24 h in a 25-cm2 flask and then adding 300 μg of EMS/ml. The medium was changed 24 h later, and the cells were incubated for a further 4–6 days before selection in BrdU.
Fluctuation Tests.
A culture was grown in HAT medium to remove any BrdUR and 6-TGR cells. 102 or 103 cells were inoculated into wells containing MEM. When cells were nearly confluent, 10 populations were trypsinized, counted, and plated in BrdU or 6-TG at 104 to 4 × 105 cells per plate, depending on the strain used. Viability was determined by plating 300–600 cells in MEM. The method of the median was used to determine the rates of resistance to BrdU or 6-TG (18).
Cell Transformation.
Plasmid pCD12 was kindly provided by Frank Maley. It includes the complete dCMP deaminase gene (19). This was subcloned as a SmaI–XhoI fragment between the PvuII–XhoI sites of pCEP4X. This is a modified form of the original pCEP4 plasmid (Invitrogen), which lacks the origin of replication, kindly provided by Zheng-Zhou Xu. It contains a cytomegalovirus promoter and a gene for hygromycin resistance. Fifteen micrograms of pCD/CEPX was used per experiment with 3 × 106 permeabilized cells. Cells were permeabilized with a Bio-Rad Gene Pulser, as previously described (15). Transformed cells were selected in MEM containing 400–800 μg/ml hygromycin (Boehringer Mannheim).
5-Methyl-dCMP Deaminase Assay.
Cells (2 × 107) were harvested, pelleted, and washed twice with isotonic saline. The pellet was suspended in 1 ml of 20 mM Tris⋅HCl buffer (pH 7.4) and disrupted by sonication. A supernatant extract was prepared by centrifugation (1 min at 14,000 rpm). Protein was determined by the A260/280 absorption procedure. The enzyme assay is based on the procedure of Maley (20). The reaction mixture contained 0.02 M Tris⋅HCl (pH 8)/2 nM MgCl2/0.04 mM dCTP/0.15 mM 5-methyl-dCMP. Optical density at 290 nm was measured using H2O as a blank. Ten microliters of supernatant extract was added to 490 μl of reaction mix, and absorption was recorded every 15 min through a time course of 90 min. All biochemicals were obtained from Sigma.
Labeling, Isolation, and Hydrolysis of DNA.
5-[3H]Methyl-dCMP (5.2 Ci/mmol) and 5-[3H]methyl-dC (5.9 Ci/mmol, purity 97% in each case) were obtained from Moravek Biochemicals (Brea, CA). 5-[3H]methyl-dCMP was also treated with calf intestinal phosphatase to remove the 5′-phosphate residues. Subconfluent cells were grown in MEM in 10-cm dishes and exposed for 24 h to 5-[3H]methyl-dC (from either source; radioactivity in the range 25–50 μCi/ml). Cells were washed twice with phosphate-buffered saline, lysed in 0.5% SDS in the presence of 0.3 M NAOH, and incubated for 1 h at 65°C to degrade RNA. The NAOH was neutralized with HCl, and an equal volume of 0.5 M Tris·HCl (pH 7.6) was added. Proteinase was added to a final concentration of 0.1 mg/ml, and the solution was again incubated at 65°C for 1 h. The tritium-labeled DNA was precipitated with 10% trichloroacetic acid (final concentration) and washed with cold 5% trichloroacetic acid and 70% ethanol. Then, the DNA was hydrolyzed to bases with 70% perchloric acid at 100°C for 1 h (21). The hydrolysate was neutralized with KOH, and the potassium perchlorate was removed by centrifugation.
HPLC Analysis.
The hydrolysate was separated by HPLC liquid chromatography on a Waters model 626/616LC fitted with a Partisil 10-μm SCX (4.6 mm × 15.0 cm) column (22). The bases were eluted at 1 ml/min with 0.05 mM potassium phosphate buffer (pH 3.5) at ambient temperature. The elution was monitored by UV absorption at 280 nm, and fractions were collected and counted with a Beckman LS3801 liquid scintillation counter using Aquasol as scintillant (6 ml). In each chromatographic run, a mixture of thymine, cytosine, and 5-methylcytosine was used as a standard. The standards identified the peaks in the hydrolysate and therefore the exact relationship between the counted fractions and the elution of thymine, cytosine, and 5-methylcytosine.
RESULTS
Growth of Cells in HAM Medium.
Aminopterin blocks the conversion of dUMP to TMP by thymidylate synthetase, so that DNA synthesis is dependent on an exogenous supply of thymidine. 5-Methyl-dC can substitute for thymidine provided the nucleoside or nucleotide deaminase is present (23) (Fig. 1). It was found that CHO K1 cells grow only slowly on HAM medium containing the normal level of 5-methyl-dC (5 μg/ml). Cells treated with the demethylating agent 5-aza-CR produced derivatives that grew well on HAM medium. These were designated HAM+, and the original strain is HAMsl. We also found that long-term selection of K1 HAMsl cells in HAM medium also gives rise to HAM+ cells. These HAM+ cells grow significantly more slowly than K1 HAMsl in normal MEM, and it was found that long-term growth of HAM+ cells in MEM leads to the re-emergence of HAMsl derivatives (results not shown). We have also shown that BrdUR derivatives of both strains, which are deficient in the enzyme thymidine kinase (TK−), grow in HAM media at the same rate as the original TK+ strain (Table 1). This demonstrates that the growth in HAM is largely or entirely due to 5-methyl-dCMP deaminase and not to 5-methyl-dC deaminase (Fig. 1). Indeed, our results are consistent with a complete absence of 5-methyl-dC deaminase activity. Also, HAM+ cells are inhibited by bromodeoxycytidine, an analog of 5-methyl-dC, whereas HAMsl cells are resistant (24). This would be expected if BrdCMP is deaminated to BrdUMP and incorporated into DNA.
Table 1.
The growth phenotypes of HAM strains with or without resistance to BrdU
| Strain | Growth on supplemented media
|
|||
|---|---|---|---|---|
| HAT | HAM | BrdU | BrdC | |
| K1 HAMsl | + | sl | − | + |
| K1 HAMsl BrdUR | − | sl | + | + |
| HAM+ | + | + | − | vsl |
| HAM+ BrdUR | − | + | + | vsl |
| HAM− | + | − | − | + |
| HAM−BrdUR | − | − | + | + |
+, vigorous growth; −, no growth; sl, slow growth in HAM medium containing 5 μg/ml 5-methyl-dC; vsl, very slow growth in medium containing 500 μg/ml or 1 mg/ml BrdC.
Isolation of the HAM− Strain.
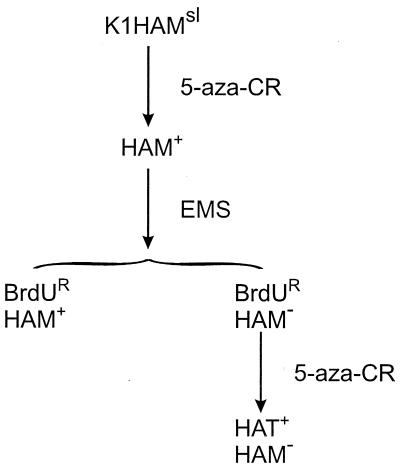
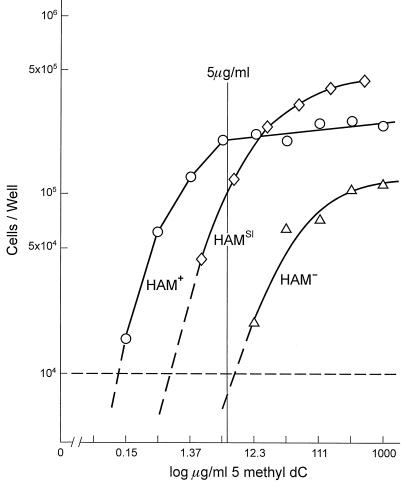
To obtain BrdUR derivatives of HAM+ cells, they were treated with the potent mutagen EMS. Among an initial batch of BrdUR isolates, one was found that was unable to grow in standard HAM medium. This isolate was initially designated HAM−, used in another report of these results (24), which we retain for convenience. However, we now know that both HAM− and HAMsl cells respond to higher concentrations of 5-methyl-dC in HAM medium and also that both can convert 5-methyl-dCMP to TMP (see below). The HAM− BrdUR isolate was reactivated with 5-aza-CR to obtain a HAT+ BrdUS derivative (see Fig. 2), which had an unaltered lack of growth in standard HAM medium containing 5 μg/ml 5-methyl-dC. [Note that both spontaneous and mutagen-induced BrdUR isolates can be reactivated by 5-azaCR because they contain a silent copy of the TK gene (25).] The response of HAMsl, HAM+, and HAM− cells to different levels of 5-methyl-dC in HAM medium is shown in Fig. 3. The difference in the response of HAM+ and HAM− to 5-methyl-dC is about 100-fold.
Figure 2.
Origins of the strains used in this study. EMS-induced BrdU-resistant strains are reactivable by 5-aza-CR because the starting strain, K1, has a silent copy of the TK gene (25).
Figure 3.
One-step growth of HAM+, HAMsl, and HAM− cells in HAM medium containing 10% dialyzed serum. Wells containing 104 cells in 1 ml of medium were counted after 4 days of incubation. The thin central vertical line indicates the standard concentration of 5-methyl-dC in HAM medium. In these experiments, the concentration of 5-methyl-dC was sequentially reduced (from 0.5 or 1.0 mg/ml) by 1:3 dilutions. Approximately 100 times less 5-methyl-dC is required by HAM+ cells to produce the same amount of growth of HAM− cells.
Epigenetic Instability of HAM− Cells.
We had previously shown by fluctuation tests that K1 HAMsl cells spontaneously produce BrdUR TK− derivatives at a rate of 6.0 × 10−5 (25). The K1 strain contains one active TK+ gene and one silent TK− gene (in other words, it is hemizygous), so the rate of appearance of TK−/TK− derivatives during normal growth can be taken as a measure of de novo methylation and inactivation of the single TK+ gene. HAM− strains produce BrdUR derivatives at a rate that is 200-fold higher than K1, as shown in Table 2. We have also shown that HAM+ produces BrdUR derivatives at a very low rate, approximately 50-fold less frequently than HAMsl. Thus, the difference in stability between HAM+ and HAM− is 10,000-fold.
Table 2.
Spontaneous resistance to BrdUR (TK−) and 6-TG (HPRT−) in HAMsl, HAM+, and HAM− strains
| Phenotype | Resistance to
|
|
|---|---|---|
| BrdU | 6-TG | |
| K1 HAMsl | 6.0 × 10−5 | ≈10−6* |
| HAM+ | ≈10−6† | <10−6* |
| HAM− | 1.2 × 10−2 | 4.6 × 10−3 |
The values are rates per cell division determined by fluctuation tests (see Materials and Methods).
6-TGR colonies in K1 are mainly mutations (15) and a comparable number may occur in HAM+, but this was not determined.
Of 10 populations (3.2 × 105 viable cells), only 3 produced at least one colony, so the median cannot be estimated. The value given is the frequency (scoring each of the populations with BrdUR colonies as one), which is higher than the median.
Cells resistant to 6-TG are HPRT− and therefore cannot grow in either HAT or HAM medium. We previously showed that rare spontaneous TGR derivatives are not reactivable by 5-aza-CR, so they are probably due to standard mutations. However, treatment of permeabilized cells with 5-methyl-dCTP produced 6-TGR derivatives at a greatly increased frequency, and these were all reactivable by 5-aza-CR (15). The evidence suggests that there is only one copy of the X-linked HPRT+ gene in CHO K1 cells, that the original inactive copy has been lost, and that the single active copy can be inactivated either by mutation or by methylation. HAM− cells produce 6-TGR HPRT− derivatives at a rate of 3.5 × 10−3 per cell division, which is about 4000-fold higher than HAMsl K1 (Table 2). In the latter strain, the rate is too low to measure by a fluctuation test, but it is approximately 10−6, which is comparable with the frequency seen in many other cell strains. Similarly, the HAM+ strain is too stable to measure either the frequency or rate of 6-TGR derivatives during normal growth. A total of 23 6-TGR colonies of independent origin were isolated in the HAM− strain. These were all unable to grow in HAT medium, as expected for HPRT− strains. All of these were reactivated to the HAT+ phenotype by 5-aza-CR using our semi-quantitative assay (25). This strongly indicates that they are epimutations due to DNA methylation. The instability of the HAM− strain does not apply to the reactivation of silenced genes. BrdUR or TGR isolates produced colonies on HAT medium at a frequency (≈10−5) comparable with CHO K1.
HAM− cells do not grow significantly more slowly than K1 HAMsl cells, although their plating efficiency is lower (about 50%, compared with 70% for HAMsl). This is surprising because the two nonessential genes we can easily examine are both inactivated at a high rate, and it might be expected that essential genes would also be inactivated. We know that the gene-silencing events are independent because 6-TGR BrdUR double-resistant cells arise in the HAM− strain at the expected frequency of ∼10−5.
We do not know the frequency of EMS-induced HAM− strains. It should be noted that the single HAM− isolate we have was derived from HAM+ BrdUS cells, and the initial screen was made on BrdUR isolates (Fig. 2). Because the HAM− strain is very unstable for the change BrdUS → BrdUR and the parent HAM+ strain is very stable (Table 2), there is a strong selection for HAM− derivatives (approximately 104-fold).
Activity of 5-Methyl-dCMP Deaminase.
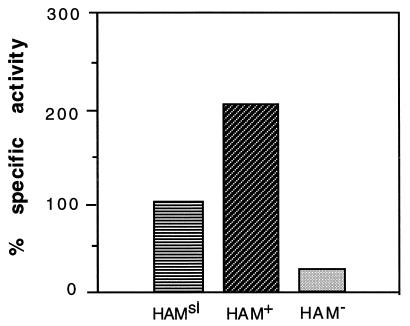
The activity of dCMP deaminase can be measured by the drop in absorption at 290 nm, when the substrate is converted to the product dUMP (20). The same assay can also be used for 5-methyl-dCMP deaminase. The results show that there is an increased level of activity in HAM+ cells, compared with HAMsl cells, and a greatly reduced activity in HAM− cells (Fig. 4).
Figure 4.
The specific activities of 5-methyl-dCMP deaminase in cell-free extracts of HAMsl (normalized to 100%), HAM+, and HAM− strains. The ratio of HAM+/HAM− activities is 8.9, and this is similar in other determinations. Boiled extracts had no activity.
Uptake of 5-[3H]Methyl-dC into the DNA of HAM− Cells.
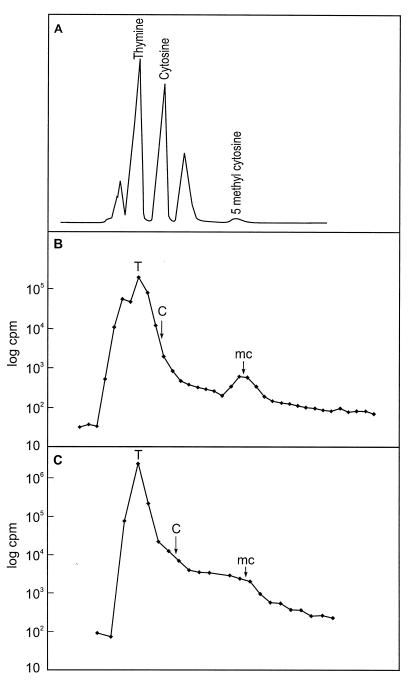
An attempt was made to measure the incorporation of 5-[3H]methyl-dCMP into DNA using permeabilized HAM− cells. Very few counts were obtained, but substantial counts were obtained when cells were incubated in the presence of 5-[3H]methyl-dC. The labeled DNA was extracted, purified, and hydrolyzed (see Materials and Methods) to the five free bases, which can be separated on HPLC (22). The counts were primarily in the thymine peak, showing that some deamination of 5-methyl-dCMP to TMP must be occurring. However, the HAM− strain also incorporated a significant number of counts into 5-methyl-C using either [3H]methyl-dC or 5-[3H]methyl-dCMP converted to the nucleoside with alkaline phosphatase. A typical result is shown in Fig. 5B. When the same experiment was carried out with the starting strain HAMsl, no discernible peak was seen in the fractions containing 5-methyl-C (Fig. 5C), even though in this experiment the counts in thymine were about 10 times higher than in the profile in Fig. 5B. There was the same lack of counts in the 5-methyl-C fractions in the HAM+ strain (results not shown).
Figure 5.
The incorporation of 5-[3H]methyl-dC into DNA in HAM− cells. (A) The separation of DNA bases by HPLC after hydrolysis. The thymine, cytosine, and 5-methylcytosine peaks are identified from a mixture of standards under the same conditions (22). The eluted fractions are collected and counted. An experiment with HAM− (B) and with wild-type HAMsl (C) cells is shown.
An Attempt to Complement the HAM− Strain.
Characterization of dCMP deaminase showed that it could use 5-methyl-dCMP as a substrate (26, 27). The dCMP deaminase has been cloned from HeLa cells (19), so the question arises whether this gene would complement the HAM− phenotype. A plasmid containing the dCMP gene with a cytomegalovirus promoter and a hygromycin resistance (hygR) gene was transfected into CHO HAM− recipient cells. Several hygR colonies were isolated and tested for their growth on HAM medium and their epigenetic instability. The frequency of BrdUR and 6-TGR colonies was unaltered, as was the poor growth on HAM medium (results not shown). dCMP deaminase is an important, but not essential, enzyme for TMP synthesis during normal growth on unsupplemented MEM medium. It is quite possible that all three strains we studied retain this enzyme, in which case complementation would not be seen (see Discussion). Because the enzyme is reported to use 5-methyl-dCMP as substrate (26, 27), it is possible that transfectants arising from the integration of multiple copies of the gene, with an increased level of dCMP deaminase, might complement the HAM− phenotype.
DISCUSSION
It is well known that transformed permanent cell lines can silence genes by DNA methylation, and it is widely assumed that this is due to a de novo DNA methyltransferase activity (5–7, 28). An alternative possibility, which we have now tested, is that these cells can misincorporate 5-methyl-dCMP arising from the ongoing repair of spontaneous damage in DNA. There are very good reasons why this should never happen in normal diploid cells, but transformed cells are known to have severe abnormalities in intermediary metabolism, including the regulation of pyrimidine pathways (14). In many cases there is very significantly increased enzyme activity, so that an inactive pathway that is blocked in normal cells could be activated or induced in transformed cells. Previous attempts to measure the uptake of labeled 5-methyl-dC into DNA have given negative results, but the biochemical methods used are relatively insensitive (9–11). The epigenetic silencing of a gene in a transformed cell line is a fairly rare event, and it could be triggered by a very low level of misincorporation of 5-methyl-dCMP.
We have obtained a derivative of K1 CHO cells that has the following properties. (i) It has a reduced level of 5-methyl-dCMP deaminase, as shown by low enzyme activity in cell-free extracts and its poor growth on HAM medium. (ii) Cells incubated in the presence of 5-[3H]methyl-dC misincorporate a significant proportion of label into 5-methyl-C in DNA, with the majority of counts appearing in thymine. (iii) It produces a very high frequency of epimutations, that is, gene-silencing events that can subsequently be reversed by the demethylating agent 5-aza-CR.
In earlier experiments, we showed that incubation of permeabilized cells in the presence of 5-methyl-dCTP induces a significant frequency of epimutations at three tested loci (15). Moreover, in detailed molecular studies of the APRT gene, we showed that the silenced gene had a high proportion of methylated CpG doublets in the promoter region (29). It is very unlikely that all this methylation is due to the incorporation of 5-methyl-dCTP. It is more likely that an initial low level of incorporation of 5 methyl-dCTP in some way triggers further methylation events, which lock the gene into a stable inactive state. The same may be true of the HAM− strain; a low level of misincorporation of 5-methyl-dCMP (via the di- and triphosphonucleotides) is sufficient to produce a dramatic increase in epimutations. It is also possible that K1 HAMsl silences genes at a lower frequency through uptake of 5-methyl-dCMP into DNA, but we have not demonstrated this biochemically.
In support of the de novo methylase hypothesis, it was shown that simian virus 40 (SV40)-transformed human fibroblasts with an artificially increased level of DNA methyltransferase activity also had an increased rate of methylation of CpG islands compared with cells with lower enzyme activity (30). We have also examined SV40-transformed human fibroblasts. Of two characterized cell lines, MRC-5V2 has a more extreme transformed phenotype (31). We have found that this strain grows very slowly in HAM medium, in fact at a rate that is comparable with the CHO HAM− strain, and it has reduced 5-methyl-dCMP deaminase activity in comparison with normal fibroblasts. This strain is also surprisingly unstable, as it produces 6-TGR HPRT− colonies at a frequency that is at least 100-fold higher than the parent untransformed MRC-5 strain (24). These observations suggest that SV40-induced transformation can lead to the misincorporation of 5-methyl-dCMP into DNA, but of course it may well be that de novo methylation can also be brought about by abnormal DNA methyltransferase activity.
At present, we have little information about the altered gene activities in the three CHO strains we have used in this study, but we know that the HAM− strain is not complemented by a plasmid with the dCMP deaminase gene. It is probable that the deamination of 5-methyl-dCMP is a very important pathway in mammalian cells to prevent the incorporation of this nucleotide (via the di-and triphosphates) into DNA. We propose that another deaminase exists, in addition to dCMP deaminase, that is either specific for or has high activity on 5-methyl-dCMP. It is probable the so-called wild-type CHO K1 cell line has a methylated gene for this enzyme, as it can be activated by 5-aza-CR to the HAM+ strain, which has high 5-methyl-dCMP deaminase activity (Figs. 3 and 4). Treatment of these cells with the mutagen EMS produced the HAM− strain. We therefore suggest that the HAM− cells can grow slowly on HAM medium and still convert most 5-methyl-dCMP to TMP (Fig. 5) because it retains dCMP deaminase activity. Further analysis, including complementation tests in hybrids, is required to determine the activities and status of the genes coding for dCMP deaminase and our proposed second enzyme.
The second mechanism that could prevent the formation of 5-methyl-dCTP and its incorporation into DNA is the proposed absence of a kinase that acts on 5-methyl-dCMP to convert it to 5-methyl-dCDP (9–11) and then to 5-methyl-dCTP. However, our results with the HAM− strain strongly indicates that there is not a complete absence of kinase activity, since we observe significant uptake of 5-[3H]methyl-dC into DNA (Fig. 5B). The results are consistent with a trickle of misincorporated 5-methyl-dCTP, arising from 5-methyl-dCMP, which is sufficient to silence genes by methylation. [The kinase converting diphosphonucleotides to triphosphonucleotides is known not to be specific (12, 13).]
Our observations suggest that 5-methyl-dCMP deaminase activity has a very important role in preventing the misincorporation of 5-methyl-dCMP into DNA. If this is so, then the loss of the enzyme, or a reduction in its activity, may have serious consequences for the cell, not least the inactivation of tumor suppressor genes. Indeed, the gene coding for 5-methyl-dCMP deaminase can itself be regarded as a candidate tumor suppressor gene. Its down-regulation could be an early event in tumorigenesis that can lead to a cascade of later heritable epigenetic changes in gene activity. In the establishment of the CHO K1 cell line, 5-methyl-dCMP deaminase activity is reduced, and, for whatever reason, these cells grow more rapidly than the HAM+ derivative with increased enzyme activity. They also produce epimutations about 50-fold more frequently than the HAM+ strain. Key features of transformed cells are their karyotypic instability, their mutability in many instances, and also their epigenetic instability (32). Chromosome changes and mutations in tumor suppressor genes are very well known, but there is much to be learned about alterations in DNA methylation associated with gene silencing or activation.
Acknowledgments
We thank Dr. Frank Maley and Dr. Zheng-Zhou Xu for providing plasmids and Richard Paulin for his help in constructing the plasmid used in our DNA transformation experiments. We also thank Jenny Young and Ewa Mylka for preparing the text and figures.
ABBREVIATIONS
- 5-methyl-C
5-methylcytosine
- 5-methyl-dC
5-methyldeoxycytidine
- 5-methyl-dCMP
5-methyldeoxycytidine monophosphate
- 5-aza-CR
5-azacytidine
- EMS
ethyl methanesulfonate
- BrdU
bromodeoxyuridine
- 6-TG
6-thioguanine
- HAT
MEM containing hypoxanthine, aminopterin, and thymidine
- HAM
MEM containing hypoxanthine, aminopterin, and 5-methyldeoxycytidine
- TK
thymidine kinase
- HPRT
hypoxanthine phosphoribosyltransferase
- CHO
Chinese hamster ovary
References
- 1.Kochanek S, Toth M, Dehmel A, Renz D, Doerfler W. Proc Natl Acad Sci USA. 1990;87:8830–8834. doi: 10.1073/pnas.87.22.8830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Behn Krappa A, Holker I, Sandaradura de Silva U, Doerfler W. Genomics. 1991;11:1–7. doi: 10.1016/0888-7543(91)90095-v. [DOI] [PubMed] [Google Scholar]
- 3.Achten S, Behn Krappa A, Jucker M, Sprengel J, Holker I, Schmitz B, Tesch H, Diehl V, Doerfler W. Cancer Res. 1991;51:3702–3709. [PubMed] [Google Scholar]
- 4.Kochanek S, Radbruch A, Tesch H, Renz D, Doerfler W. Proc Natl Acad Sci USA. 1991;88:5759–5763. doi: 10.1073/pnas.88.13.5759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baylin S B. Science. 1997;277:1948–1949. doi: 10.1126/science.277.5334.1948. [DOI] [PubMed] [Google Scholar]
- 6.Baylin S B, Herman J G, Graff J R, Vertino P M, Issa J-P. Adv Cancer Res. 1988;72:141–196. [PubMed] [Google Scholar]
- 7.Lengauer C, Krizler K W, Vogelstein B. Proc Natl Acad Sci USA. 1997;94:2545–2550. doi: 10.1073/pnas.94.6.2545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lindahl T. Nature (London) 1993;362:709–715. doi: 10.1038/362709a0. [DOI] [PubMed] [Google Scholar]
- 9.Jekunen A, Puukka M, Vilpo J. Biochem Pharmacol. 1983;32:1165–1168. doi: 10.1016/0006-2952(83)90265-4. [DOI] [PubMed] [Google Scholar]
- 10.Vilpo J A, Vilpo L M. Mutat Res. 1993;286:217–220. doi: 10.1016/0027-5107(93)90186-j. [DOI] [PubMed] [Google Scholar]
- 11.Vilpo J A, Vilpo L M. Somatic Cell Mol Genet. 1995;21:285–288. doi: 10.1007/BF02255783. [DOI] [PubMed] [Google Scholar]
- 12.Roisin M P, Kepes A. Biochim Biophys Acta. 1978;526:418–428. doi: 10.1016/0005-2744(78)90133-x. [DOI] [PubMed] [Google Scholar]
- 13.Munoz-Dorado J, Inouye S, Inouye M. J Biol Chem. 1990;265:2707–2712. [PubMed] [Google Scholar]
- 14.Weber G. Cancer Res. 1983;43:3466–3492. [PubMed] [Google Scholar]
- 15.Holliday R, Ho T. Somatic Cell Mol Genet. 1991;17:537–542. doi: 10.1007/BF01233618. [DOI] [PubMed] [Google Scholar]
- 16.Nyce J. Somatic Cell Mol Genet. 1991;17:543–550. doi: 10.1007/BF01233619. [DOI] [PubMed] [Google Scholar]
- 17.Holliday R, Ho T. Somatic Cell Mol Genet. 1995;21:215–218. doi: 10.1007/BF02254772. [DOI] [PubMed] [Google Scholar]
- 18.Lea D E, Coulson C A. J Genet. 1949;49:264–285. doi: 10.1007/BF02986080. [DOI] [PubMed] [Google Scholar]
- 19.Weiner K X B, Weiner R S, Maley F, Maley G F. J Biol Chem. 1993;268:12983–12989. [PubMed] [Google Scholar]
- 20.Maley F. Methods Enzymol. 1967;12:170–182. [Google Scholar]
- 21.Wilson V L, Jones P A. Cell. 1983;32:239–246. doi: 10.1016/0092-8674(83)90514-7. [DOI] [PubMed] [Google Scholar]
- 22.Singer J, Stellwagen R H, Roberts-Ems J, Riggs A D. J Biol Chem. 1977;252:5509–5513. [PubMed] [Google Scholar]
- 23.Chan T, Long C, Green H. Somatic Cell Genet. 1975;1:81–90. doi: 10.1007/BF01538733. [DOI] [PubMed] [Google Scholar]
- 24.Holliday, R. & Ho, T. (1998) Mutat. Res.400, in press. [DOI] [PubMed]
- 25.Holliday R, Ho T. New Biol. 1990;2:719–726. [PubMed] [Google Scholar]
- 26.Scarano E, Bonaduce L, De Petrocellis B. J Biol Chem. 1962;237:3742–3751. [Google Scholar]
- 27.Maley G F, Maley F. J Biol Chem. 1964;239:1168–1176. [PubMed] [Google Scholar]
- 28.Holliday R. Science. 1987;238:163–170. doi: 10.1126/science.3310230. [DOI] [PubMed] [Google Scholar]
- 29.Paulin R P, Ho T, Balzer H J, Holliday R. Genetics. 1998;149:1081–1088. doi: 10.1093/genetics/149.2.1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vertino P M, Chiu Yen R-W, Gao J, Baylin S B. Mol Cell Biol. 1996;16:4555–4565. doi: 10.1128/mcb.16.8.4555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huschtscha L I, Holliday R. J Cell Sci. 1983;63:77–99. doi: 10.1242/jcs.63.1.77. [DOI] [PubMed] [Google Scholar]
- 32.Lindahl T, editor. Genetic Instability in Cancer, Cancer Surveys. Vol. 28. Plainview, NY: Cold Spring Harbor Lab. Press; 1996. [PubMed] [Google Scholar]