Abstract
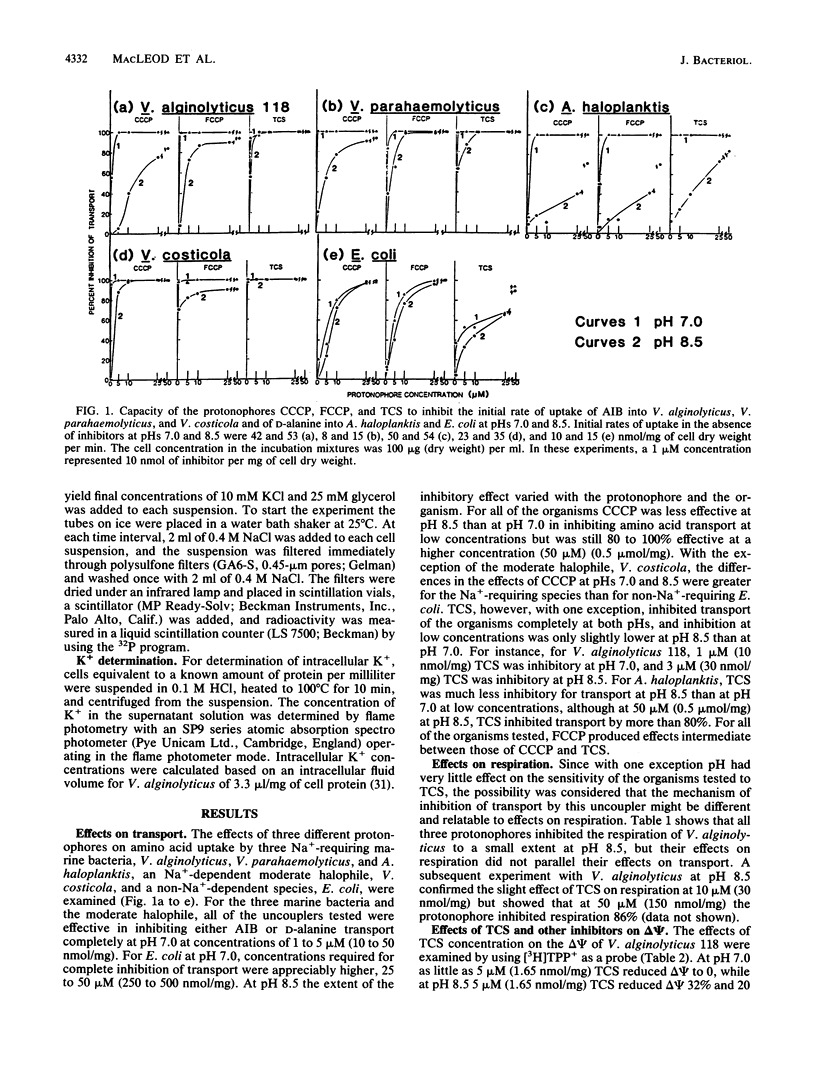
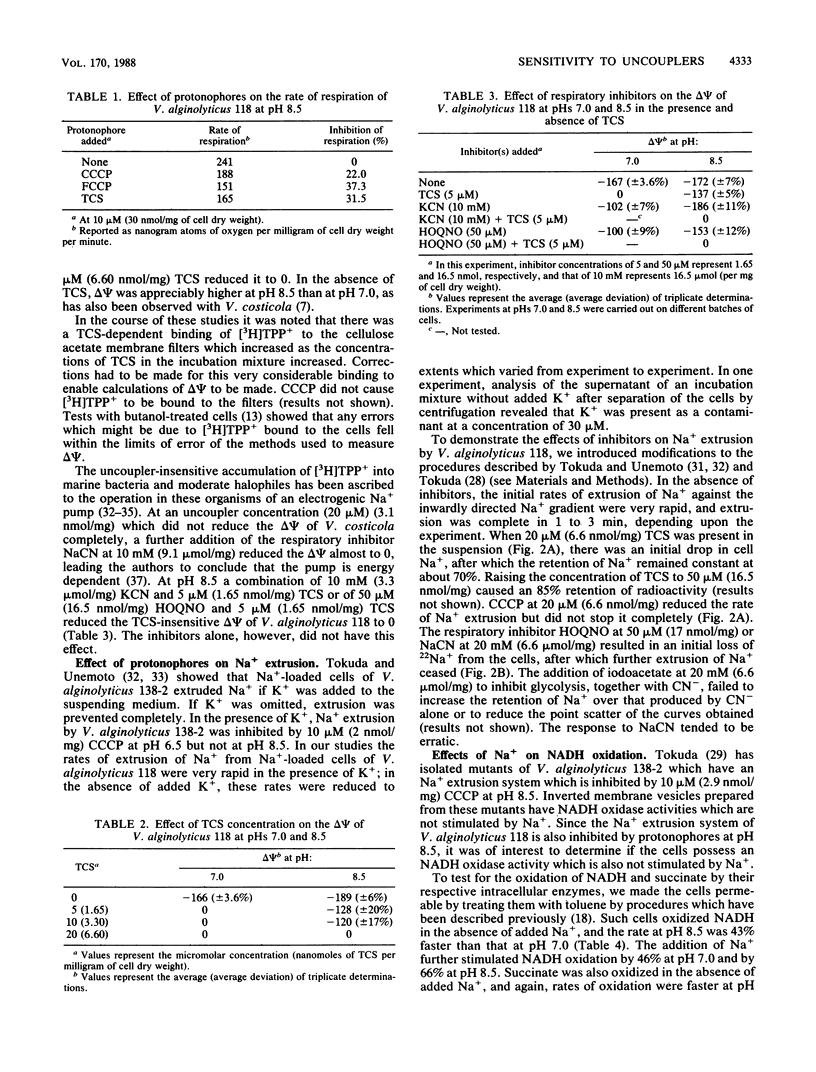
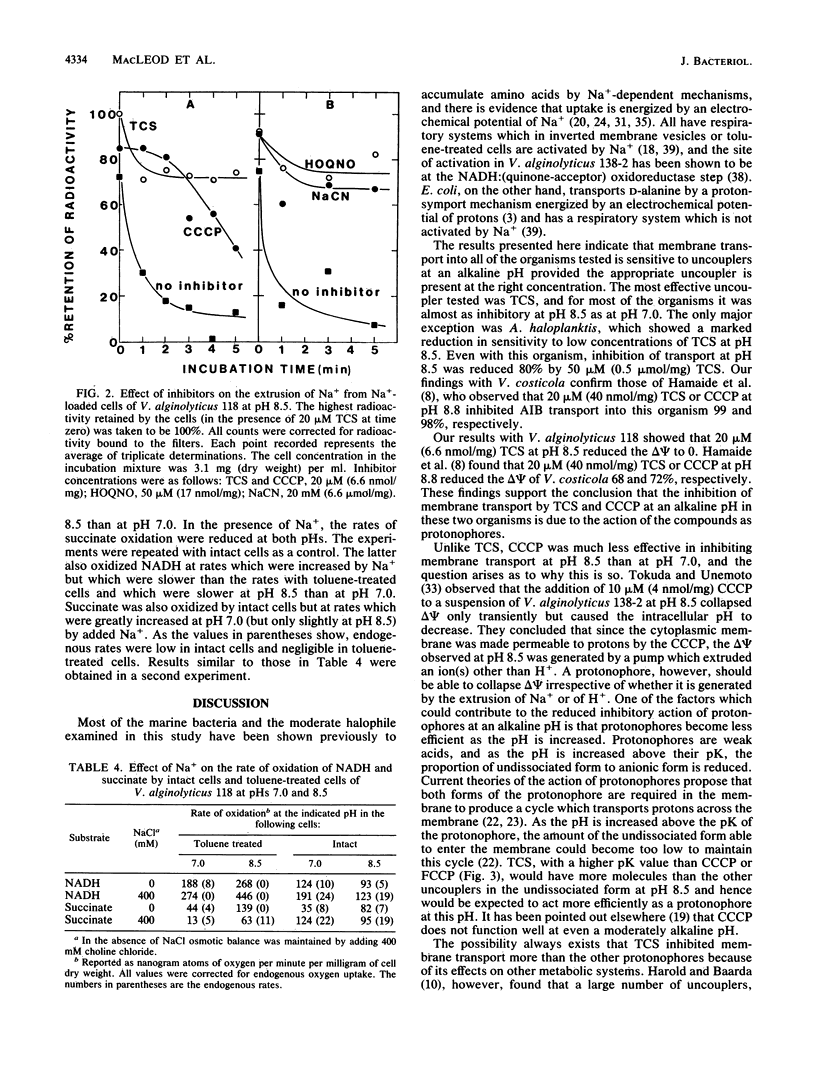
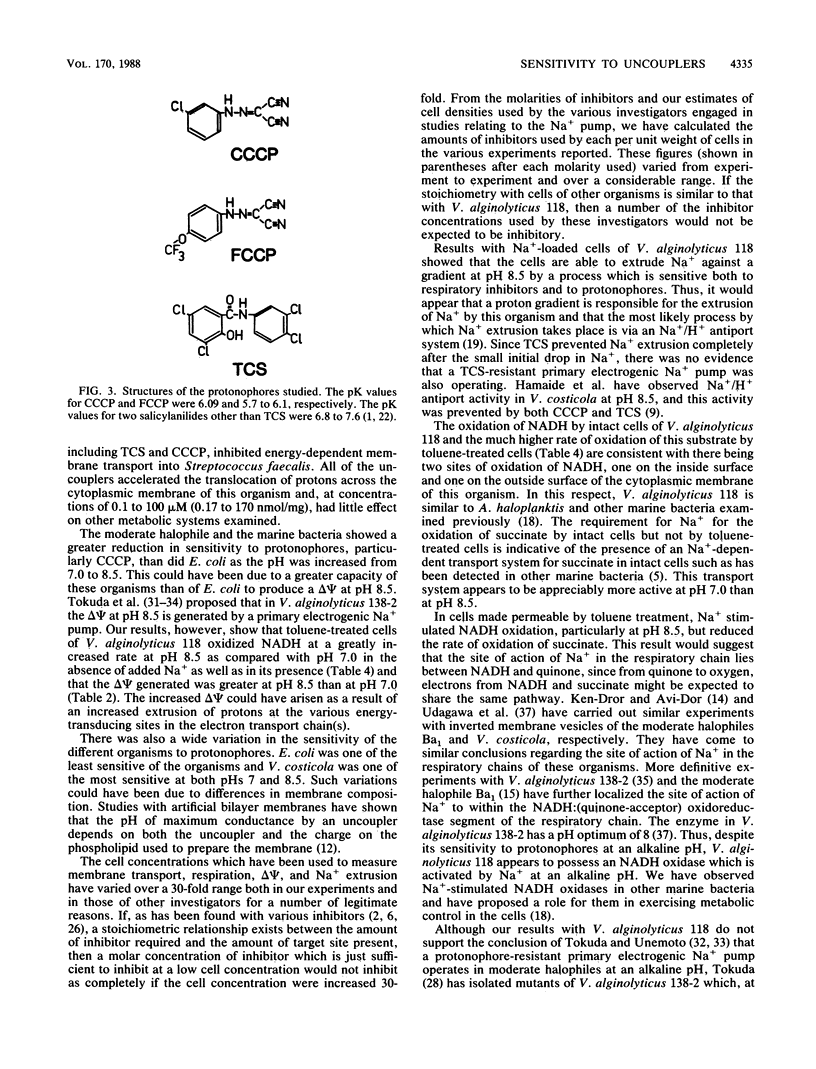
The inhibitory effects of uncouplers on amino acid transport into three marine bacteria, Vibrio alginolyticus 118, Vibrio parahaemolyticus 113, and Alteromonas haloplanktis 214, into a moderate halophile, Vibrio costicola NRC 37001, and into Escherichia coli K-12 were found to vary depending upon the uncoupler tested, its concentration, and the pH. Higher concentrations of all of the uncouplers were required to inhibit transport at pH 8.5 than at pH 7.0. The protonophore carbonyl cyanide m-chlorophenylhydrazone showed the greatest reduction in inhibitory capacity as the pH was increased, carbonyl cyanide p-trifluoromethoxyphenylhydrazone showed less reduction, and 3,3',4',5-tetrachlorosalicylanilide was almost as effective as an inhibitor of amino acid transport at pH 8.5 as at pH 7.0 for all of the organisms except A. haloplanktis 214. Differences between the protonophores in their relative activities at pHs 7.0 and 8.5 were attributed to differences in their pK values. 3,3',4',5-Tetrachlorosalicylanilide, carbonyl cyanide m-chlorophenylhydrazone, 2-heptyl-4-hydroxyquinoline-N-oxide, and NaCN all inhibited Na+ extrusion from Na+-loaded cells of V. alginolyticus 118 at pH 8.5. The results support the conclusion that Na+ extrusion from this organism at pH 8.5 occurs as a result of Na+/H+ antiport activity. Data are presented indicating the presence in V. alginolyticus 118 of an NADH oxidase which is stimulated by Na+ at pH 8.5.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bakker E. P., Arents J. C., Hoebe J. P., Terada H. Surface potential and the interaction of weakly acidic uncouplers of oxidative phosphorylation with liposomes and mitochondria. Biochim Biophys Acta. 1975 Jun 17;387(3):491–506. doi: 10.1016/0005-2728(75)90088-2. [DOI] [PubMed] [Google Scholar]
- Beechey R. B., Roberton A. M., Holloway C. T., Knight I. G. The properties of dicyclohexylcarbodiimide as an inhibitor of oxidative phosphorylation. Biochemistry. 1967 Dec;6(12):3867–3879. doi: 10.1021/bi00864a033. [DOI] [PubMed] [Google Scholar]
- Berger E. A., Heppel L. A. Different mechanisms of energy coupling for the shock-sensitive and shock-resistant amino acid permeases of Escherichia coli. J Biol Chem. 1974 Dec 25;249(24):7747–7755. [PubMed] [Google Scholar]
- Dibrov P. A., Kostryko V. A., Lazarova R. L., Skulachev V. P., Smirnova I. A. The sodium cycle. I. Na+-dependent motility and modes of membrane energization in the marine alkalotolerant vibrio Alginolyticus. Biochim Biophys Acta. 1986 Jul 23;850(3):449–457. doi: 10.1016/0005-2728(86)90113-1. [DOI] [PubMed] [Google Scholar]
- Droniuk R., Wong P. T., Wisse G., Macleod R. A. Variation in Quantitative Requirements for Na for Transport of Metabolizable Compounds by the Marine Bacteria Alteromonas haloplanktis 214 and Vibrio fischeri. Appl Environ Microbiol. 1987 Jul;53(7):1487–1495. doi: 10.1128/aem.53.7.1487-1495.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ESTABROOK R. W. Observations on the antimycin A inhibition of biological oxidations. I. Stoichiometry and pH effects. Biochim Biophys Acta. 1962 Jul 2;60:236–248. doi: 10.1016/0006-3002(62)90399-2. [DOI] [PubMed] [Google Scholar]
- Hamaide F., Kushner D. J., Sprott G. D. Proton circulation in Vibrio costicola. J Bacteriol. 1985 Feb;161(2):681–686. doi: 10.1128/jb.161.2.681-686.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamaide F., Kushner D. J., Sprott G. D. Proton motive force and Na+/H+ antiport in a moderate halophile. J Bacteriol. 1983 Nov;156(2):537–544. doi: 10.1128/jb.156.2.537-544.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamaide F., Sprott G. D., Kushner D. J. Energetics of sodium-dependent alpha-aminoisobutyric acid transport in the moderate halophile Vibrio costicola. Biochim Biophys Acta. 1984 Jul 27;766(1):77–87. doi: 10.1016/0005-2728(84)90219-6. [DOI] [PubMed] [Google Scholar]
- Harold F. M., Baarda J. R. Inhibition of membrane transport in Streptococcus faecalis by uncouplers of oxidative phosphorylation and its relationship to proton conduction. J Bacteriol. 1968 Dec;96(6):2025–2034. doi: 10.1128/jb.96.6.2025-2034.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashket E. R., Blanchard A. G., Metzger W. C. Proton motive force during growth of Streptococcus lactis cells. J Bacteriol. 1980 Jul;143(1):128–134. doi: 10.1128/jb.143.1.128-134.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ken-Dror S., Avi-Dor Y. Regulation of respiration by Na+ and K+ in the halotolerant bacterium, Ba1. Arch Biochem Biophys. 1985 Nov 15;243(1):238–245. doi: 10.1016/0003-9861(85)90792-1. [DOI] [PubMed] [Google Scholar]
- Ken-Dror S., Lanyi J. K., Schobert B., Silver B., Avi-Dor Y. An NADH:quinone oxidoreductase of the halotolerant bacterium Ba1 is specifically dependent on sodium ions. Arch Biochem Biophys. 1986 Feb 1;244(2):766–772. doi: 10.1016/0003-9861(86)90645-4. [DOI] [PubMed] [Google Scholar]
- Ken-Dror S., Preger R., Avi-Dor Y. Functional characterization of the uncoupler-insensitive Na+ pump of the halotolerant bacterium, Ba1. Arch Biochem Biophys. 1986 Jan;244(1):122–127. doi: 10.1016/0003-9861(86)90100-1. [DOI] [PubMed] [Google Scholar]
- Ken-Dror S., Shnaiderman R., Avi-Dor Y. Uncoupler-stimulated Na+ pump and its possible role in the halotolerant bacterium, Ba. Arch Biochem Biophys. 1984 Mar;229(2):640–649. doi: 10.1016/0003-9861(84)90197-8. [DOI] [PubMed] [Google Scholar]
- Khanna G., DeVoe L., Brown L., Niven D. F., MacLeod R. A. Relationship between ion requirements for respiration and membrane transport in a marine bacterium. J Bacteriol. 1984 Jan;157(1):59–63. doi: 10.1128/jb.157.1.59-63.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krulwich T. A. Na+/H+ antiporters. Biochim Biophys Acta. 1983 Dec 30;726(4):245–264. doi: 10.1016/0304-4173(83)90011-3. [DOI] [PubMed] [Google Scholar]
- Kushner D. J., Hamaide F., MacLeod R. A. Development of salt-resistant active transport in a moderately halophilic bacterium. J Bacteriol. 1983 Mar;153(3):1163–1171. doi: 10.1128/jb.153.3.1163-1171.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LENNOX E. S. Transduction of linked genetic characters of the host by bacteriophage P1. Virology. 1955 Jul;1(2):190–206. doi: 10.1016/0042-6822(55)90016-7. [DOI] [PubMed] [Google Scholar]
- McLaughlin S. G., Dilger J. P. Transport of protons across membranes by weak acids. Physiol Rev. 1980 Jul;60(3):825–863. doi: 10.1152/physrev.1980.60.3.825. [DOI] [PubMed] [Google Scholar]
- Mitchell P. Chemiosmotic coupling in oxidative and photosynthetic phosphorylation. Biol Rev Camb Philos Soc. 1966 Aug;41(3):445–502. doi: 10.1111/j.1469-185x.1966.tb01501.x. [DOI] [PubMed] [Google Scholar]
- Skulachev V. P. Membrane-linked energy transductions. Bioenergetic functions of sodium: H+ is not unique as a coupling ion. Eur J Biochem. 1985 Sep 2;151(2):199–208. doi: 10.1111/j.1432-1033.1985.tb09088.x. [DOI] [PubMed] [Google Scholar]
- Tatum E. L., Lederberg J. Gene Recombination in the Bacterium Escherichia coli. J Bacteriol. 1947 Jun;53(6):673–684. doi: 10.1128/jb.53.6.673-684.1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokuda H. Isolation of Vibrio alginolyticus mutants defective in the respiration-coupled Na+ pump. Biochem Biophys Res Commun. 1983 Jul 18;114(1):113–118. doi: 10.1016/0006-291x(83)91601-7. [DOI] [PubMed] [Google Scholar]
- Tokuda H., Nakamura T., Unemoto T. Potassium ion is required for the generation of pH-dependent membrane potential and delta pH by the marine bacterium Vibrio alginolyticus. Biochemistry. 1981 Jul 7;20(14):4198–4203. doi: 10.1021/bi00517a038. [DOI] [PubMed] [Google Scholar]
- Tokuda H. Sodium translocation by NADH oxidase of Vibrio alginolyticus: isolation and characterization of the sodium pump-defective mutants. Methods Enzymol. 1986;125:520–530. doi: 10.1016/s0076-6879(86)25041-7. [DOI] [PubMed] [Google Scholar]
- Tokuda H., Sugasawa M., Unemoto T. Roles of Na+ and K+ in alpha-aminoisobutyric acid transport by the marine bacterium Vibrio alginolyticus. J Biol Chem. 1982 Jan 25;257(2):788–794. [PubMed] [Google Scholar]
- Tokuda H., Unemoto T. A respiration-dependent primary sodium extrusion system functioning at alkaline pH in the marine bacterium Vibrio alginolyticus. Biochem Biophys Res Commun. 1981 Sep 16;102(1):265–271. doi: 10.1016/0006-291x(81)91516-3. [DOI] [PubMed] [Google Scholar]
- Tokuda H., Unemoto T. Characterization of the respiration-dependent Na+ pump in the marine bacterium Vibrio alginolyticus. J Biol Chem. 1982 Sep 10;257(17):10007–10014. [PubMed] [Google Scholar]
- Tokuda H., Unemoto T. Growth of a marine Vibrio alginolyticus and moderately halophilic V. costicola becomes uncoupler resistant when the respiration-dependent Na+ pump functions. J Bacteriol. 1983 Nov;156(2):636–643. doi: 10.1128/jb.156.2.636-643.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokuda H., Unemoto T. Na+ is translocated at NADH:quinone oxidoreductase segment in the respiratory chain of Vibrio alginolyticus. J Biol Chem. 1984 Jun 25;259(12):7785–7790. [PubMed] [Google Scholar]
- Tsuchiya T., Shinoda S. Respiration-driven Na+ pump and Na+ circulation in Vibrio parahaemolyticus. J Bacteriol. 1985 May;162(2):794–798. doi: 10.1128/jb.162.2.794-798.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udagawa T., Unemoto T., Tokuda H. Generation of Na+ electrochemical potential by the Na+-motive NADH oxidase and Na+/H+ antiport system of a moderately halophilic Vibrio costicola. J Biol Chem. 1986 Feb 25;261(6):2616–2622. [PubMed] [Google Scholar]
- Unemoto T., Hayashi M., Hayashi M. Na+-dependent activation of NADH oxidase in membrane fractions from halophilic Vibrio alginolyticus and V. costicolus. J Biochem. 1977 Nov;82(5):1389–1395. doi: 10.1093/oxfordjournals.jbchem.a131826. [DOI] [PubMed] [Google Scholar]
- Unemoto T., Hayashi M. NADH: quinone oxidoreductase as a site of Na+-dependent activation in the respiratory chain of marine Vibrio alginolyticus. J Biochem. 1979 Jun;85(6):1461–1467. doi: 10.1093/oxfordjournals.jbchem.a132474. [DOI] [PubMed] [Google Scholar]



